|
| |||||
|
| ||||||||
|
| ||||||||
Home  Publications Publications |
|
|
Note to the Reader Visual Neuroscience (1991) 6: 403–406 © copyright http version by Robert W. Williams
Print Friendly Abstract One of the most characteristic features of primate retina is the high
density of rods throughout the periphery. In humans, for example, rods
outnumber cones 25 to 1 in mid-peripheral retina (Curcio et al., 1990). This
factor contributes to the superior light sensitivity of peripheral retina.
The idea that rods maintain their dominance out to the extreme periphery is
deeply entrenched (e.g., Polyak, 1941). However, little effort has actually
been expended on the retinal edge, and even a brief survey of standard
references reveals intriguing discrepancies. For instance, Schwalbe (1874)
stated that density of rods fell sharply at the ora serrata, and Østerberg
(1935) mentioned, almost in passing, that the density of cones rose at three
sites near the ora on the nasal side of a single retina he studied. Techniques introduced recently by Curcio and colleagues (1987) now make
it practical to rapidly quantify the structure of the mosaic at any location
in a number of glycerin-embedded retinal wholemounts. The method combines
high-resolution video-enhanced differential interference contrast optic with
a video-overlay system. This combination makes it possible to determine the
structure of the mosaic around the entire perimeter of the retina. Retinas from 9 humans were studied. The average age of material was 61
years (28-82 years). Retinas and the pars planae of the ciliary body were
dissected and flattened between coverslips in a mixture of glycerin, water,
and polyvinyl alcohol (Heimer & Taylor, 1974). There is no appreciable
tissue shrinkage. Two retinas were reacted using polyclonal antibodies
directed against cone opsins (see Lerea et al., 1989, for details). A total
of 281 sites (6200 µm2 each) completely free of
microcysts were analyzed in retinas from 8 individuals. Sites were
quantified at 3500× using differential interference contrast optics, analog
and digital video enhancement, and a long working distance oil-immersion
objective from the edge of retina inward 2 mm (Wikler et al., 1990). Parts
of the periphery were also embedded in plastic and semithin sections were
cut to study lamination and cell morphology. As described in the classical literature (Schwalbe, 1874; Salzmann, 1912)
and as confirmed by the analysis of transverse plastic sections, all layers
of the retina extend out to the extreme periphery. Layers are thinner;
ganglion cells are widely spaced (Stone & Johnston, 1981). Microcysts are
common within the outer layers of retina in the older human material (Ochi,
1927). Such microcystic regions were avoided. This report only covers the
photoreceptor mosaic. Distinguishing between rods and cones was not appreciably more difficult
at the retinal rim than elsewhere in retina (Fig.
1, A and B). Cone inner segments are typically four times larger than
those of neighboring rods (70 ± 16 µm2 vs. 18.2 ±
5.9 µm2). Cone inner segments at the retinal edge
are somewhat smaller than those even 50 microns farther in. The size
distribution of rods and cones shows no overlap at any eccentricity.
The validity of morphological criteria used to classify rod and cones was
corroborated using antibodies directed against cone opsins (Lerea et al.,
1989). A subset of outer segments in the periphery was heavily labeled (Fig.
1., C and D). With antibodies directed against red and green opsins, up
to 90% of large inner segments (putative cones) could be traced to labeled
outer segments in well-reacted parts of the extreme periphery in humans. In
many cases, however, cone inner segments could not be traced to labeled or
unlabeled outer segments. The outer segment is especially sensitive to
mechanical damage in postmortem immersion-fixed tissue, and it is also
probable that some outer segments are short or have undergone senescent
change (Salzmann, 1912). Small inner segments (rods) could never be traced
to labeled outer segments. The blue cone opsin antibody labeled a very small
number of outer segments in the extreme periphery. This immunological work
unequivocally validates structural criteria used to distinguish rod and cone
inner segments. The peak packing density of cones at the edge of the retina consistently
ranges between 10,000 and 15,000/mm2. This is 3 to
5 times higher than values in the mid-periphery (Curcio, 1990). The average
within the outer 500 micron band is about 8,000 cones/mm2.
Within this band the ratio of surface area occupied by cones and rods is
about 8:1 (Fig. 2). This ratio is pertinent because it takes into account
the much larger area—hence, light-gathering capacity—of cone inner segments,
and provides a rough estimate of the proportion of photons initially
captured by the two receptor systems. The cone-enriched rim contains as many
as 250,000 cones. In comparison, human fovea contains in the neighborhood of
75,000 cones (Curcio et al. 1990, their fig. 6).
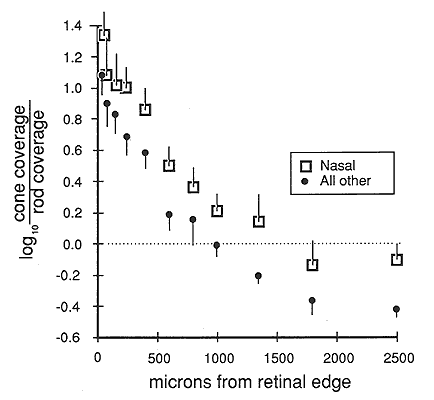
Fig. 2. Plot of the shift in the structure of the human
photoreceptor mosaic from rod dominance (right side) to cone dominance near
the retinal edge (left side). The photoreceptor coverage ratio used in this
figure is the ratio between the surface area occupied by cones and that
occupied by rods, here on a logarithmic y-axis. More than 1500 µm from the
edge of the retina, rods typically occupy twice as much of the retina as do
cones (log coverage ratio = -0.3). At a distance of 1000 microns, the retina
is divided almost equally (log of the coverage ratio = 0), and within 250
microns of the edge, cones typically occupy 90% to 95% of the mosaic (log
coverage ratio of 0). The fovea would be far to the right at about 22,000
microns. Data points are averages from 99 nasal sites, and 182 sites
distributed equally in temporal, dorsal, and ventral quadrants from 8 human
retinas. For purpose of analysis, values in excess of log 2 were treated as
log 2. Only sites free of microcysts were analyzed. Such sites are prominent
in aged material and can be readily recognized and avoided by focusing at
the level of the outer plexiform layer. They appear as large tissue-free
vacuoles. Bars on data points indicate the standard error of the mean.
The cone-enriched rim is most pronounced in the nasal and upper nasal
part of the retina. In humans, the density along the nasal margin is
typically 10,000/mm2 (range: 6,000/mm2
to 15,000/mm2), whereas the average in other parts
of the retina is typically 8,000/mm2 (range:
3,000/mm2 to 12,000/mm2).
Rods are present throughout most of the cone-enriched belt, but often at
remarkably low densities. Within 500 microns of the edge the average rod
density in humans is under 10,000/mm2, and along
the nasal margin it is not uncommon to find fields in which densities are
under 3,000/mm2 (Fig.
1A). In contrast, rod densities over most of the mid-periphery are well
above 40,000/mm2. Over what distance does the transition from rod to cone dominance occur?
Within 1-2 mm of the edge, cone densities begin to rise (Fig. 2). The
crossover point—the distance at which the retinal surface is partitioned
equally between rods and cones—is located 1500 microns from the edge in the
nasal sector and about 1000 microns elsewhere (Fig. 2). From this point
moving outward, the change is rapid, and over a distance of only 800
microns, the ratio of the area covered by cones to that covered by rods
increases from 1:1 to 10:1. Within 250 microns of the edge, cones occupied
10 times more area that rods. The remarkable switch from rod- to
cone-dominated retina is as abrupt along the radial axis as that along the
rim of the fovea. Individual variation within the cone belt is substantial but is no higher
that that which characterizes human and monkey fovea (Curcio et al., 1990;
Wikler et al., 1990). However, there was unexpected variation in the
structure of the mosaic at neighboring sites in periphery. For example, in
an extreme case, one field had cone and rod densities of 11,000/mm2
and 4,700/mm2, respectively, whereas an adjacent field had cone
and rod densities of 6,600/mm2 and 12,200/mm2. Each human retina had elevated cone densities, particularly along the
nasal periphery. This region that corresponds to a 5- to 10-degree-wide
swath of the extreme lateral part of the visual field (Donders, 1877; Drasdo
& Fowler, 1974). In contrast, the elevation of cones was not as marked in
non-nasal parts of retina. These non-nasal regions of the retinal margin are
shielded by the eyebrow, the cheek, and the side of the nose. Consequently,
peripheral vision at maturity does not normally extend much beyond 65
degrees in these directions, and a large peripheral sector, including much
of the cone-enriched rim in temporal and ventral quadrants, does and
probably do not have a role in vision at maturity. While it is far from
proof, the fact that the most prominent part of the cone-enriched rim is
optically aligned with the extreme temporal periphery suggests that these
cones have functional significance. In this context, it is worth noting that
I have also observed a prominent increase in cone coverage in the nasal
periphery of several other Old World primates (R.W. Williams, unpublished).
Unlike the fovea, the periphery of the retina is primarily engaged in
detection, not resolution. In this context, there are several reasons why a
band of cones along the nasal edge of the retina might be advantageous. One
possibility is that these cones are used to detect objects crossing into the
visual field on the basis of color. Ferree and Rand (1927) demonstrated
convincingly that color fields extend to the extreme periphery, provided
that the stimulus is sufficiently large (5 degree diameter), but to date no
abrupt change in color function, corresponding to the rim of cones, has been
noted at the edge of the visual field. A second possible advantage is that
cone responses are not saturated in bright light (Aguilar & Stiles, 1954).
Consequently, this band of cones may ensure that peripheral stimuli evoke a
strong response even in broad daylight. A third possible advantage is that
the time response of cones is at least two times faster than that of rods
even at moderate levels of illumination at which both types function
(Conner, 1982), and cones in the periphery appear to have particularly rapid
response times (Tyler, 1985). A fast-acting alert mechanism would have
considerable adaptive value, particularly in primate species in which the
lateral coverage of the visual field has been compromised by a large
binocular field. Finally, it is also possible that the three-fold increase
in the packing density of cones compensates for the steep drop in image
magnification in the extreme periphery (Drasdo & Fowler, 1974) thereby
maintaining a more nearly constant density of cones per steradian. This
reduction in magnification in the periphery also increases the intensity of
illumination at the edge, compensating somewhat for the pupillary vignetting.
Because it is so technically demanding, psychophysical tests of retinal
function have not yet been carried out in the extreme periphery of humans.
It is clear that such work will be essential to test whether the cone
enriched rim has a particular role in human vision. [Motivated in part by this report, Mollon and colleagues (1998) looked
for psychophysical correlates of the cone-enriched rim. They were unable to
find any evidence for changes either in flicker detection or color naming
for stimuli persented at very high eccentricity to two male subjects (26 and
53 years of age). While their results fail to support a functional role for
the cone enriched rim, they note that "it remains possible that a
(functional) discontinuity might be detected in younger subjects, or by a
different measure."] From a comparative view point it is of interest to note that the edge of
the retina is highly specialized in some birds and lizard. In fact, in some
species there is even be a second, far temporal fovea (Fite & Lister, 1981)
related to frontal vision. This demonstrates that the optical quality of
peripheral retina is not invariably poor. Recent work on the growth of retina provides the basis for a model of the
development of the cone-enriched rim. In mammals, rods are generated long
after cones (e.g., LaVail et al., 1991). Rods appear to intercalate between
cones as the retina expands (Fernald, 1988). There is a good possibility
that the rim of the retina is stiff and does not expand much (Lia et al.,
1987; Kelling et al, 1989) thereby preserving an initially high
concentration of cones. Acknowledgements I thank the donors and their families for providing tissue and Dr. C.
Lerea for generously providing antibodies. Human tissue was obtained with
the help of K. Allen of the Lions Eye Banks, Seattle, Washington. My thanks
to D. Turner, H. Zhou, and P. Nguyen for technical assistance; and D.
Goldowitz, C. Johnson, and K. Graehl for stimulating discussion and comment
on the manuscript. This work was supported by NEI 6627 and the University of
Tennessee Center for Neuroscience. References Aguilar, M. & Stiles, W.S. (1954) Saturation of the rod mechanism of the
retina at high levels of stimulation. Opt. Acta 1, 59–65. Conner, J.D. (1982) The temporal properties of rod vision. J. Physiol.
332, 139–155. Curcio, C.A., Packer, O. & Kalina, R.E. (1987) A whole mount method for
sequential analysis of photoreceptors and ganglion cells in a single retina.
Vision Res. 27, 9–15. Curcio, C.A., Sloan, K.R., Kalina, R.E. & Hendrickson, A.E. (1990) Human
photoreceptor topography. J. Comp. Neurol. 292, 497–523. Donders, F.C. (1877) Die Grenzen des Gesichtsfeldes in Beziehung zu denen
der Netzhaut. Albrecht. v. Graef's Arch. f. Ophthal. 23, 255–280. Drasdo, N. & Fowler, C.W. (1974) Non–linear projection of the retinal
image in a wide–angle schematic eye. Br. J. Ophthalmol. 58, 709–714. Fernald, R.D. (1988) Retinal rod neurogenesis. In Development of the
Vertebrate Retina. Finlay, B.L. & Sengelaub D.R., eds. New York: Plenum
Press. Ferree, C.E. & Rand, G. (1927) Effect of size of stimulus on size and
shape of color fields. Amer. J. Ophthal. 10, 399–411. Fite, K.V. & Lister, B.C. (1981) Bifoveal vision in Anolis lizards. Brain
Behav. Evol. 19, 144–154. Heimer, G.V. & Taylor, C.E.D. (1974) Improved mountant for
immunofluorescence preparations. J. Clin. Path. 27:254–256. Greeff, R. (1931) Mikroskopische Anatomie des Sehnerven und der Netzhaut.
In Handbuch der Gesamten Augenheilkunde, 2nd ed. vol. 1, pt. 2 chapt. 5, p.
113, fig. 36, Berlin: Verlag J. Springer. Kelling, S.T, Sengelaub, D.R. Wikler, K.C. & Finlay, B.L. (1989) Visual
Neurosci. 2, 117–120. LaVail, M.M., Rapaport, D.H. & Rakic, P. (1991) Cytogenesis in the monkey
retina. J. Comp. Neurol. 309:86–114. Lerea, C.L., Bunt–Milam, A.K. & Hurley, J.B. (1989) Alpha transducin is
present in blue–, green–, and red–sensitive cone photoreceptors in the human
retina.Neuron 3, 367–376. Lia, B., Williams, R.W. & Chalupa, L.M. (1987) Formation of retinal
ganglion cell topography during prenatal development. Science 236, 848–851.
Mollon, J.D., Regan, B.C., Bowmaker, J.K. (1998) What is the function of
the cone–rich rim of the retina? Eye 12, 548–552. Ochi, S. (1927) So–called
cystic degeneration in the peripheral retina. Am. J. Ophthal. 10, 161–163.
Østerberg, G. (1935) Topography of the layer of rods and cones in the
human retina. Acta Ophthalmol. 13 [Suppl.] 6, 1–103. Polyak, S.L (1941) The Retina. Chicago: University of Chicago Press. Salzmann, M. (1912) The Anatomy and Histology of the Human Eyeball in the
Normal State. Its Development and Senescence. Chicago: University of Chicago
Press. Schwalbe, G. (1874) Mikroscopische Anatomie des Sehnerven, der Netzhaut
und das Glaskoerpers. In Handbuch der Allgemeinen Augenheilkunde, Vol. 1,
Graefe, A. & Saemisch, T., eds. Leipzig: Verlag W. Engelmann. Stone, J. & Johnston, E. (1981) The topography of primate retina: A study
of the human, bushbaby and New– and Old–World monkeys. J. Comp. Neurol. 196,
205–223. Tyler, C.W. (1985) Analysis of visual modulation sensitivity. II.
Peripheral retina and the role of photoreceptor dimensions. J. Opt. Soc. Am.
2, 393–398. Wikler, K.C., & Rakic, P. (1990) Distribution of photoreceptor subtypes
in the retina of diurnal and nocturnal primates. J. Neurosci. 10, 3390–3401.
Wikler, K.C., Williams, R.W. & Rakic, P. (1990) Photoreceptor mosaic:
Number and distribution of rods and cones in the rhesus monkey retina. J.
Comp. Neurol. 297, 499–508. Since 11 August 98
|
Neurogenetics at University of Tennessee Health Science Center
| Print Friendly | Top of Page |
Mouse Brain Library | Related Sites | Complextrait.org