|
| ||||||
|
| ||||||||
|
| ||||||||
Home  Publications Publications |
|
|
Print Friendly Autosomal dominant optic
atrophy (OPA1) is the most common form of hereditary optic atrophy in
humans, with an incidence of 1:50,000 (165500; GDB 1995). A recent study has
localized OPA1 to Chromosome (Chr) 3 between q28-qter (Eiberg et al.
1994). OPA1 is characterized by a loss of visual acuity, deficits in
color vision, and scotomas of varying size (Eliott et al. 1993). Retinas of
patients with OPA1 have a reduction in the number of retinal ganglion
cells and a decrease in myelin content in the optic nerves, chiasm, and
tracts (Johnston et al. 1979; Kjer et al. 1983). Neurons that are in the
main target of the retinal ganglion cell projection, the dorsal lateral
geniculate nucleus, are also atrophic (Kjer et al. 1983). Other cell
populations in the retina of humans with OPA1 appear to be normal
(Johnston et al. 1979; Kjer et al. 1983). OPA1 is a dominant
mutation, but the expression of the phenotype is highly variable both within
and among families (Kline and Glaser 1979). The loss of visual acuity and
the atrophy of the optic nerves often varies between right and left sides
(Kline and Glaser 1979; Kjer et al. 1983). Recently, we have identified a striking abnormality in
optic nerves of mice that are heterozygous for the spontaneous mutation
belly spot and tail (Bst). Bst is a semi-dominant, homozygous lethal
mutation that arose in the inbred strain C57BLKS (BKS; previously denoted
C57BL/Ks). Heterozygous mice have a kinky tail, white feet, and a white spot
at the ventral midline. In approximately 50% of the Bst/+ mice, there
is a reduction or a complete absence of the pupillary light reflex in one or
both eyes (Rice et al. 1993). This neurological phenotype is associated with
a unilateral or bilateral atrophy of the optic nerves. As in humans with
OPA1, the severity of the atrophy of the optic nerves is highly
variable--ranging from a slight reduction in the number of ganglion cell
axons in one optic nerve to a complete elimination of both optic nerves. The
surface area of the retina and the appearance of the inner and outer nuclear
layers are qualitatively normal (Rice et al. 1993). The Bst locus has been mapped previously as the
distal-most locus of a three-point cross in relation to Igl1 (immunoglobin
lambda-I) and md (mohaganoid) on Chr 16 (Epstein et al. 1986). Harris
et al. (1989) subsequently mapped Bst in a two-point cross with
Sod1 (superoxide dismutase- 1). Collectively, these studies place the
Bst locus 25 to 42 cM distal to the centromere and proximal to Sod1.
This region of mouse Chr 16 is conserved in human Chr 3 (Reeves and
Citron 1994). Given the marked phenotypic similarity of retinal phenotypes
between OPA1 and Bst and the chromosomal homology, we have
generated a higher resolution map of Bst on Chr 16 using an
intraspecific backcross. F1 hybrids were produced by crossing BKS-Bst/+
females to AKR males. The (BKS x AKR)F1-Bst/+ males and
females were crossed to wildtype BKS, generating a total of 157 backcross
progeny. These progeny were phenotyped for Bst by inspecting the tail
for kinks and the belly for white hairs. Genomic DNA was isolated for the
analysis of simple sequence length polymorphisms (SSLP). A total of 11
primer pairs recognizing SSLP loci were used to map Bst more
precisely. PCR reactions were carried out as described by Dietrich and
associates (1992) with two modifications: (i) the Taq DNA polymerase
concentration was doubled (0.5U/rxn), and (ii) 30, instead of 25 cycles,
were run. PCR products were separated on 7% nondenaturing polyacrylamide
gels and stained with ethidium bromide. Recombination frequencies were
analyzed with the program Map Manager v2.51 (Manly and Elliott 1991). The results of the haplotype analysis establish the
following order among loci with distances in cM ± standard error:
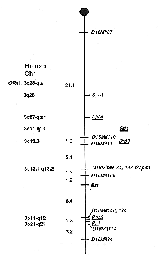
D16Mit87-21.1 ± 6.6-D16Mit110-1.3 ± 1.3-D16Mit11-5.1 ±
1.8-D16Mit138, D16Mit84, D16Mit39-1.3 ± 0.9-D16Mit168-1.9 ±
1.1-Bst-6.4 ± 2.0-D16Mit14O, D16Mitl74-1.3 ± 0.9-D16Mit114-3.2
± 1.4-D16Mit94. Thus, according to the placement of these markers on
the consensus map for mouse Chr 16 (Reeves and Citron 1994), Bst lies
approximately 39 cM from the centromere (Fig. I). The percentage of mutants in the backcross (33.8%) is
less than the 50% expected for a dominant Mendelian trait. There are two
possible explanations. First, the penetrance of the mutation may be
decreased in the backcross progeny (see Epstein et al. 1986). If the paucity
of Bst/+ mutants in the backcross is reduced because of incomplete
penetrance, then flanking markers should still exhibit normal 1:1
segregation. However, in our backcross, markers linked to the Bst
locus exhibit a marked segregation distortion, consistent with our Bst
genotype assignments (Fig. 2). Second, the in utero survival rate of the
Bst heterozygote may be compromised. The mean litter size is reduced
in the backeross compared with the intercross (6.0 vs. 7.8), and the
percentage of Bst/+ mutants is reduced in backcross litters (Table
I). Furthermore, Bst heterozygotes on the C57BLKS inbred background
are not as viable (mean litter size = 4.6) as heterozygous mice whose
genetic background is a mosatc of AKR and C57BLKS alleles. Thus, the
disparity in the BKS alleles in the backcross is probably the result of the
in utero elimination of Bst/+ mutants. In a related study, we have
found in utero death associated with a high incidence (about 30%) of
exencephaly in crosses using Bst/+ mice (Rice et al. 1993). We have mapped the Bst locus to a region of mouse
Chr 16 that is conserved in human Chr 3. Some of the loci on human Chr 3
that are also located on mouse Chr 16 include: apolipoprotein d (APOD),
pituitary transcription factor (PIT1), dopamine D3 receptor (DRD3),
preprosomatostatin (SMST), stefin 1 (STFI), R0S2, and
growth-associated protein (GAP43; Fig. I). Gap43 has recently
been shown to be important for the growth of retinal ganglion cell axons at
the optic chiasm in the mouse (Strittmatter et al. 1995). However, on the
basis of the current map positions, GAP43 (which maps to human Chr
3q13.1-q13.2; Naylor et al. 1994) does not appear to be a candidate for
OPA1 although it cannot be ruled out as a candidate for the Bst
mutation. OPA1 and Bst have many similarities. Both
mutations are inherited as dominant phenotypes with variable expressivity,
and both appear to target retinal ganglion cells. It has been postulated
that the OPA1 mutation results in a primary degeneration of the cells
in the ganglion cell layer and, subsequently, in atrophy of the optic nerves
(Kjer et al. 1983). The decrease in retinal ganglion cells in the Bst/+
mouse is evident as early as the day of birth (Rice et al. 1993) and before
the onset of naturally occurring ganglion cell death (Williams et al. 1990). The mechanisms responsible for the congenital decrease
to ganglion cell number in humans with the OPA1 mutation and in
Bst/+ mutant mice are unknown. Comparison of the order of homologous
loci in the mouse and human chromosomal maps for this region suggests that
OPA1 and Bst map to different regions of the conserved
segment. Therefore, they either may not be mutations in the same gene or the
gene order may differ between the mouse and human chromosome within these
conserved segments. Despite this caveat, the remarkable similarities in the
atrophy of the ganglion cell population between OPA1 and Bst
make the Bst mutant mouse a good model to study the abnormal
development of the ganglion cell population. Acknowledgments. We thank Janice Hyatt for
assistance with genomic DNA isolation. This work was supported by National
Institutes of Health (NIH) Grant NS EY-09586 (D. Goldowitz), NIH
Neuroscience training grant NS-07323 (D.S. Rice), NIH Grant PO1 RR01183, and
Cancer Core Grant CA34196 (B.S. Harris, P. Ward-Bailey, KR. Johnson, MT.
Davisson). References Dietrich, W.F., Katz, H., Lincoln, SE., Shin, H.-S.,
Friedman. J., Dracopila, N., Lander, E.S. (1992). A genetic map of the mouse
suitable for typing intraspecific crosses. Genetics 131, 423-447. Eiberg, H., Kjer, B., Kjer. P., Rosenberg, T. (1994).
Dominant optic atrophy (OPA1) mapped to chromosome 3q region. I. Linkage
analysis. Hum. Mol. Genet. 3, 977-980. Eliott, D., Traboulsi, E.I., Maumenee. I.H. (1993).
Visual prognosis in autosomal dominant optic atrophy (Kjer type). Am. J.
Ophthalmol. 115, 360-367. Epstein, R., Davisson, M.T., Lehmann, K., Akeson. E.C.,
Cohn, M. (1986). Position of Igl-1, md, and Bst loci on
chromosome 16 of the mouse. Immunogenetics 23, 78-83. GDB, Human Genome Database. (1995). The Johns Hopkins
University Bininformatics Web Server (URL:http//gdbwww.gdb.org). Green, M.C., Witham, B.A. (1991) Handbook on Genetically
Standardized JAX Mice, 4th ed. (Bar Harbor, ME: The Jackson Laboratory). Harris, B.S., Cook, S., Davisson, M.T. (1989). Mouse News
Lett. 84, 90. Johnston, P.B., Gaster, R.N., Smith, V.C., Tripathi, R.C.
(1979). A clinicopathologic study of autosomal dominant optic atrophy. Am.
J. Oph thalmol. 88, 868-875. Kjer, P., Jensen, O.A., Klinken, L. (1983).
Histopathology of eye, optic nerve and brain in a case of dominant optic
atrophy. Acta Ophthalmol. 61, 300-312. Kline, L.B., Glaser, J.S. (1979). Dominant optic atrophy.
The clinical profile. Arch. Ophthalmol. 97, 1680. Manly, K.F., Elliott, R.W. (1991). RI manager, a
microcomputer program for analysis of data from recombinant inbred strains.
Mamm. Genome 1, 123-127. Mouse Genome Database (MGD). (1995). Mouse Genuine
Informatics Project, The Jackson Laboratory, Bar Harbor, Me. World Wide Web
(URL:http//www.informatics.jax.org). Naylor, S.L., Buys, C.H.C.M., Carritt, B. (1994). Report
on the fourth annual workshop on human chromosome 3 mapping. Cytogenet. Cell
Genet. 65, 2-34. Reeves, R.H., Citron, M.P. (1994). Mouse Chromosome 16.
Mamm. Genome 5 (suppl.), S229-S337. Rice, D.S., Williams, R.W., Davisson, M.T., Harris, B.,
Goldowitz, D. (1993). A new mutant phenotype of retinal ganglion cell
disgenesis discovered in the mouse. Society of Neuroscience Abstr. 19, 51. Strittmatter, S.M., Fankhauser, C., Huang, P.L., Mashimo,
H., Fishman, M.C. (1995). Neuronal pathfinding is abnormal in mice lacking
the neuronal growth cone protein Gap-43. Cell 80, 445-452. Williams, M.A., Pinon, L.G.P., Linden, R., Pinto, L.H.
(1990). The pearl mutation accelerates the schedule of natural cell death in
the early postnatal retina. Exp. Brain Res. 82, 393-400. Williams, R.W. (1994). The portable dictionary of the
mouse genome: a personal database for gene mapping and molecular biology.
Mamm. Genome 5, 372-375. World Wide Web (URL:http//www.nervenet.org). Mammalian Genome 6: 546-548 (1995). Since 16 June 99
|
Neurogenetics at University of Tennessee Health Science Center
| Print Friendly | Top of Page |
Mouse Brain Library | Related Sites | Complextrait.org