|
| ||||||
|
| ||||||||
|
| ||||||||
Home  Publications Publications |
|
|
Note to the Reader This is a revised and annotated version of a paper first published in The Journal of Neuroscience. The quality of figures 8 and 12 (two darkfield micrographs) are improved. Two new figures have been incorporated into this revision. Annotation and additions are delimited by brackets [...]. Enlarging images. Thumbnail versions of all figures are embedded in the paper. Full-size images—under 300K—will download into a separate window when you select the thumbnails. Drag this second window to the side of the text window. In some cases, high-resolution images—usually under 600K—that match the quality of the original micrographs can be downloaded by selecting the text at the bottom the corresponding figure legend. These image files can be viewed with Adobe Photoshop, NIH Image, or equivalent. Revised HTML edition <http://www.nervenet.org/papers/SC1982.html> copyright © 1998 by Robert W. Williams
Print Friendly The Journal of Neuroscience 2:604–622 (1982)
Introduction
The retinal projection to the superior colliculus of the cat was
examined at four gestational ages, embryonic days 38, 46, 56, and 61,
using the anterograde transport of horseradish peroxidase and tritiated
leucine. Gestation in the cat averages 65 days. By the 38th day of
gestation (E38) the entire superior colliculus, both ipsilateral and
contralateral to an injection, was labeled intensely. The peroxidase label
had an extremely coarse-grained texture. As late as E46, the ipsilateral
retinal projection extended to the presumed caudal border of the
colliculus, while by E56, as in the mature cat, the ipsilateral terminal
field was largely excluded from the rostral and caudal tectal poles. As in
younger fetal material, at E56 the contralateral terminal field extended
across the entire tectal mantle. By E61 clear gaps of label were observed
in the contralateral stratum griseum superficiale. At this age patches or
bands of label were accentuated on the ipsilateral side. In double-labeled
tissue, it could be demonstrated that the ipsilateral patches corresponded
quite closely to the gaps in the contralaterally-derived label. Thus, a
few days before birth, the retinal projection to the superior colliculus
has a pattern which resembles that of the adult. During the last decade, the advent of axoplasmic transport tracing
methods has provided a wealth of data on developing retinofugal
projections in a variety of vertebrates ranging from the frog (Currie and
Cowan, 1975) to the monkey (Rakic, 1977a). Recent work has shown that the
intitial distribution of retinal ganglion cell axons in fetal and neonatal
mammals is more diffuse and extensive than can be demonstrated in the
adult (Rakic, 1977a; Cavalcante and Rocha-Miranda, 1978; Frost et al.,
1979; Land and Lund, 1979; Sanderson et al., 1982).
Materials and MethodsTimed pregnancies were obtained by exposing an estrous female to a
potent male cat for 24 hr. To ensure that the female was receptive, a
number of matings were observed. The end of the 24-hr exposure period
marked the beginning of embryonic day 1 (E1). This method of timing
conception is only accurate to within 1 day. Gestation in the domestic cat
has a duration ranging from 62 to 67 days.
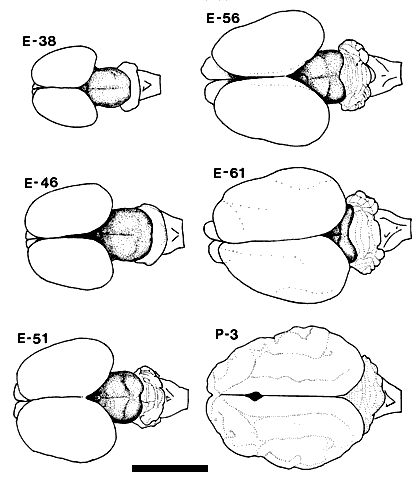
ResultsThe midbrain of the fetal cat is a conspicuous and relative large structure which extends 6.0 mm in the rostrocaudal axis by the 5th week of gestation. (Fig. 1). The tectal cleft, setting off the superior from the inferior collicular anlagen, is not easily distinguished at this age. However, if the most caudal extension of retinal ganglion cell axons is considered to mark this boundary, then as early as E38, the superior colliculus extends 2.7 ± 0.2 mm. A comparable figure for the mature superior colliculus is 5.0 ± 0.5 mm. At E38 and E46, no demarcation can be made between the rostral collicular margin and the pretectum (Fig. 2). By E56, the pretectal nuclei, especially the nucleus of the optic tract and the pretectal olivary nucleus, can be differentiated from the more dorsal fasciculi of retinal efferents [Williams and Chalupa, 1984], and at the rostral collicular margin, a portion of these efferents becomes capped dorsally by the superficial gray. At this age, then, the anterior border of the superior colliclus is well defined. Figure 1. Drawings based on photographs of the dorsal view of the fetal and early postnatal cat brain. The mesencephalon, including the superior and inferior colliculi, is clearly visible as the shaded portion of the drawings of the fetal brains. This drawing includes an E51 fetus not otherwise covered in the Results. Calibration bar, 1 cm. Injections of tracer substances were made into the eyes of 36 fetal cats ranging in age from E30 to E61. The results presented in this paper are based on the 10 fetuses (see Table 1) in which there was no retinal damage and in which fixation by perfusion was successful so that axonal transport to the superior colliculus could be demonstrated for at least one of the tracers. All photomicrographs and photomontages are identified both by the age and number of the individual cat, in parentheses, from which material was obtained.
Table 1: Summary data on cases
Table 2: Quantitative Analysis of Labeling
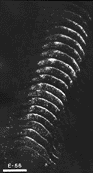
*Adult data from Graybiel 1975 As early as E30, a prominent optic chiasm is present (Anker, 1977; R.W. Williams, unpublished observation, [1983 thesis]), and it is therefore probable that optic axons have penetrated the roof of the mesencephalon at least a few days before the end of the first month of gestation. Shatz and DiBerardino (1980) have demonstrated a bilateral projection to the cat diencephalon as early as E32, and at this age Shatz (personal communication) has noted the presence of a weak contralateral retinocollicular projection. Figure 2. The retinal projection to the superior colliculus of fetus E38(5) demonstrated by the anterograde transport of HRP. The rostral pole of the superior colliculus is shown in the lowest photograph of the montage, and the caudal pole is shown in the uppermost photograph. Theleft side of the montage is contralateral to the eye which received the peroxidase injection.l Note how extensive the uncrossed projection to the superior colliculus is at this gestational age, especially in comparison with that in older fetuses (see Figs. 7 and 9). Adjacent sections are spaced approximately 150 µm apart. Calibration bar, 1 mm.
E38. In our material, a bilateral projection to the mantle of the superior colliculus anlage could be demonstrated with both tracers as early as E38. The distribution of peroxidase label in one of the three successful E38 fetuses is shown in Figure 2. A gradient of the density of the TMB chromogen through the depth of the tectal plate is evident. This plate is characterized by a very high cell population density with a maximum thickness of 300 µm. The intense label ends abruptly at the ventral margin of the plate. Medial and lateral portions of the contralateral colliculus were labeled more heavily that was the central band. This erosion of label toward the middle was even more marked on the ipsilateral side (Fig. 2, right side). In spite of these gradients in the density of label, the entire rostrocaudal extent of the contralateral tectal plate was labeled heavily.
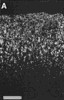
Figure 3. HRP-TMB label at E38. A. Coarse-grained peroxidase label in the caudal ipsilateral superior colliculus of fetus E38(5). B, Peroxidase-labeled growth cone in the contralateral DLGn of the same fetus. Calibration bar, 50 &mciro;m in A; 5 µm in B. Heavy coarse grained label also filled the medial and lateral portions
of the ipsilateral tectal mantle (Fig. 2, right side). As on the
contralateral side the central band was considerably less intensely
labeled. Along the anteroporterior axis the heavy label extended roughly
2.6 mm from the pretectal border to within a few hundred micrometers of
the caudal tip of the superior colliculus. The peroxidase label in the
most posterior 300 µm was not heavy, but it was nevertheless distinct. The
meager uncrossed projection to the extreme caudal pole of the E38 (5)
fetus may represent the precursor of the monocular crescent seen in mature
cats (Graybiel, 1976).
Figure 4. The distribution of label within the superior colliculus fo fetus E46(2). This series of drawings was made using a Bausch and Lomb projection microscope. Conventions are as in Figure 2. Two tiers of label are evident on the ipsilateral (right) side. Adjacent sections are spaced approximately 200 µm apart. Calibration bar, 1 mm. The contralateral autoradiographic label confirmed the picture obtained using the peroxidase method. Figure 5A, an autoradiograph through the anterior contralateral superior colliculus, demonstrates a significant change in the quality of label with depth. The dorsal 100 µm of the collicular label is dense and uniform, whereas the ventral 150 µm of label is mottled. The significance of this variation in the quality of autoradiographic and peroxidase label with depth is not known. It is possible that the mottled appearance in the deeper half of the plate is caused by (1) heavily labeled fasciculi of retinal fibers, (2) aggregates of growth cones, or (3) the particular types of retinal axons arborizing or growing in the deeper portions of the retinorecipient laminae. In a study of the prenatal development of the retionfugal projection in the rat, Lund and Bunt (1976) found that the initial complement of retinal fibers coursed tangentially along the surface of the tectum. The majority of these fibers eventually came to lie under the stratum opticum. At E38 the heaviest label was located just below the pia, whereas by E46 some of the heaviest and most coarse label was located along the ventral margin of the tectal mantle. The mottled appearance of label in far rostral sections through the E46 midbrain thus may represent the formation of the stratum opticum.
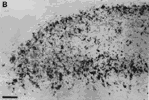
Figure 5. A, Autoradiograph showing the pattern of label in the rostral contralateral superior colliculus of fetus E46(2). This dark-field-illuminated micrograph shows the change in the quality of label with depth: the superficial label is relatively homogeneous; the deeper label is mottled. The deeper label may presage the formation of the statum opticum. B, Granularity of peroxidase label along the medial edge of the ipsilateral superior colliclus of fetus E46(2). This coronal section is from the caudal one-third of the superior colliculus. Medial is to the left. Calibration bars, 50 µm.
Figure 6. Two relatively distinct tiers of HRP-TMB label are evident in this coronal section through the ipsilateral superior colliculus of fetus E46(2). As in Figure 4 the label is lighter than background. This coronal section was taken 1.9 mm posterior to the rostal collicular border. Medial is the the left. Calibration bar, 200 µm. Even as late as E46, uncrossed label covered the entire tectal plate. This expansive projection was seen in both peroxidase and autoradiographic material. At E46 there was no indication that the undecussated retinocollicular projection had begun to retract from either the anterior or posterior poles of the superior colliculus. The reorganization that occurs between E38 and E46 suggests either the onset of the elaboration of finer axonal plexuses or the segregation of particular types of ganglion cell axons.
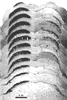
Figure 7. A photomontage of 16 sections spaced approximately 180 µm apart showing peroxidase label in the contralateral (left) and ipsilateral (right) superior colliculus of fetus E56(2). Conventions are as in Figure 2. Note the substantial change in the distribution of ipsilateral label in comparison to E38(5) (Fig. 2). The ipsilateral label is restricted tothe central region, but in distinction to the E61 material (Figs. 9 and 10), the label is still diffuse. The vertical gaps in label seen especially on the contralateral side are vascular channels. Calibration bar, 1 mm. Despite a careful examination and reconstruction of the contralateral collicular label, no sign of the representation of the contralateral optic disc, which is evident in the adult cat as an unlabeled patch (Graybiel, 1975), could be found. Since every section was saved and reacted in fetus E56(2) and every other section in fetus E56(1), it is improbable that we simply neglected such a gap. The exclusion of contralateral label from the area corresponding to the optic disc had not progressed noticeably at this gestational age.
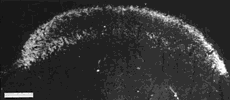
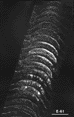
Figure 8. Montage of the ipsilateral retinal projection to the superior colliculus of fetus E56(2). The dark-field effect was obtained uisng two Polaroid type HN32 linear polarizers crossed for maximum extinction. Adjacent sections are spaced approximately 120 µm apart. Banding of the ipsilateral retinocollicular projection may be incipient at this gestational age. Calibration bar, 1 mm.
Figure 9. The retinal projection to the superior colliculus of fetus E61(4) demonstrated by the orthograde transport of HTP. Conventions are as in Figures 2 and 7. On the contralateral (left) side, areas of less intense label are found within the middle of the statum griseum sperficiale. Adjacent sections are spaced approximately 240 µm apart except between the upper two photographs of the montage between which the spacing is approximately 500 µm. Calibration bar, 1 mm. Another feature which distinguished the E61 fetal projection from that of younger fetuses was the unequivocal presence of two tiers of retinal label in the anterior one-half of the contralateral superior colliculus (Fig. 9). The upper tier was composed of a virtually unbroken sheet of very dense label 60 to 80 µm thick. This dense sheet of label directly abutted the stratum zonale but was clearly restricted to the superficial portion of the SGS (SGS-1) of Kanaseki and Sprague, 1974). This tectal layer is the major recipient of retinal input in the mature cat (Sterling, 1973; Mize, 1981). Of interest in this regard is the recent observation that focal peroxidase injections made into the upper SGS result in the retrograde labeling of small ganglion cells of the gamma class (Itoh et al., 1980). The deep tier of retinal label was 100 to 150 µm thick and extended down to the stratum opticum. This tier was largely resticted to the deep lamina of the superficial gray, SGS-3. The more sparsely labeled stratum sandwiched between these tiers corresponded to SGS-2, which at E61 received the bulk of the uncrossed retinal input. The contralateral projection to SGS-2 took the form of poorly defined bands of label extending from SGS-1 to SGS-3, with center-to-center separation ranging from 150 to 250 µm. Defining the borders of diffuse bands precisely was difficult in both autoradigraphic and peroxidase material, but their breadth ranged from 60 to 150 µm.
Figure 10. The partial segregation of crossed and uncrossed retinal fibers to the superior colliculus of fetus E61(5). Alternate sections treated for autoradiography (3H) or for the presence of peroxidase (HRP) were drawn under dark-field illumination. Peroxidase sections show the crossed retinal projection to the left, whereas the autoradiographic sections show the uncrossed projection to the left. Upper drawings are at more caudal levels of the superior colliculus. Calibration bar, 1 mm.
Figure 11. The optic disc representation gap seen in HRP-TMB-reacted tissue. A, Partial clearing of label is evident in the stratum zonale and the upper portion of stratum griseum superficiale of fetus E61(3). B, In the neonate, P0(1), the gap extends through the stratum zonale and the entire stratum griseum superficiale. Calibration bar, 50 µm for A and B.
Figure 12. Polarized light photomicrograph of the uncrossed retinal projection to fetus E61(4). Adjacent sections are spaced approximately 160 µ apart. Patches or bands of label are now clearly evident within the stratum griseum superficiale. Conventions are as in Fig. 2. Calibration bar, 1 mm. The ipsilateral retinal projection described by Graybiel (1976) and shown in her Figures 1b and 2a and b, resembles that seen in fetus E61(4). She also noted the presence of a fractured superficial sheet of label in addition to the more ventral bands in SGS-2. Thus a few days before birth the retinal projection to the superior colliculus matches that described by Graybiel (1975, 1976) and Harting and Guillery (1976) in the adult cat. In particular, at E61, as in the adult cat, the retinocollicular projection has the following attributes: (i) clear patches of label are seen in the ipsilateral superior colliculus which, in some instances, correspond to gaps in label derived from the contralateral retina; (ii) the stratum zonale is devoid of label; and (iii) the rostral and caudal margins of the ipsilateral superior colliculus are unlabeled. It is, of course, possible that the pattern of the retinocollicular projection undergoes further changes; however, any modificiations in the distribution of retinotectal fibers after birth are certainly less dramatic that those that occur during the latter half of gestation.
DiscussionThis study describes the formation of retinal projections to the
superior colliculus of the cat. We have found that by the 38th day of
gestation each retina’s projections to the superior colliculus are
partially segregated. Several days before birth the retinal projection to
the superior colliculus has a pattern similar to that of mature cats. The
retinal innervation of the ipsilateral superior colliculus appears to
involve at least three steps: (i) a generous axonal outgrowth covering the
complete tectal sheet, (ii) a retraction along the anteroposterior axis
such that the projection is excluded from both the rostral and caudal
collicular poles, and (iii) a condensation of the uncrossed terminal
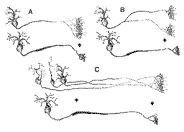
arbors into bands restricted largely to SGS-2. Figure 13. Schematics of possible causes underlying the retraction of terminal field label in the superior colliculus. Three 'before-and-after' situations are depicted. A, The telodendria of the retinocollicular axons condense to form restricted terminal fields. B, Major axons collaterals are eliminated during development. C, Retinal ganglion cell number is reduced and axon arbors of dying cells are lost.
AcknowledgementsThis research was supported inpart by the National Institutes of Health under grant 532 GM 07416. We thank Dr. Charles J. Sedgwick, Department of Veterinary Medicine, University of California, Davis, for his guidance and assistance in the surgical procedures.
ReferencesAnker RL (1977) The prenatal development of some of the visual pathways in the cat. J Comp Neurol 173:185–204.
Behan M (1981) Identification and distribution of retinocollicular terminal in the cat: An electron microscopic autoradiographic analysis. J Comp Neurol 199:1–15. Bowling, D. B., and C. R. Michael (1980) Projection patterns of single physiologically characterized optic tract fibers in cat. Nature 286: 899–902. Boycott BB, Wässle H (1974) The morphological types of ganglion cells of the domestic cat’s retina. J Physiol (Lond) 240:397–419. Brückner G, Mareš V, Biesold D (1976) Neurogenesis in the visual system of the rat. An autoradiographic investigation. J Comp Neurol 166:245–256. Cavalcante LA, Rocah–Miranda CE (1978) Postnatal development of retinogeniculate, retipretectal and retinotectalprojections in the opossum. Brain Res 146:231–248. Cleland BG, Levick WR (1974a) Brisk and sluggish concentrically organized ganglion cells in the cat’s retina. J Physiol (Lond) 240:421–456. Cleland BG, Levick WR (1974b) Properties of rarely encountered types of ganglion cells in the cat’s retina and an overall classificiation. J Physiol (Lond) 240:457–492. Cowey A, Perry VH (1980) The projection of the fovea to the superior colliculus in rhesus monkeys. Neuroscience 5:53–61. Cragg BG (1975) The development of synapses in the visual system of the cat. J Comp Neurol 160:147–166. Cunningham TJ, Mohler IM, Giordano DL (1981) Naturally occurring neuron death in the ganglion cell layer of theneonatal rat: morphology and evidence for regional correspondence with neuron death in the superior colliculus. Dev Brain Res 2:203–215. Currie J, Cowan WM (1975) The development of the retino–tectal projection in Rana pipiens. Dev Biol 46:103–119. Dreher B, Sefton AJ (1979) Properties of neurons in cat’s dorsal lateral geniculate nucleus: a comparison between medial interlaminar and laminated parts of the nucleus. J Comp Neurol 183:47–64. Frost DO, So KF, Schneider GE (1979) Postnatal development of retinal projections in Syrian hamster: a study using autoradiographic and anterograde degeneration techniques. Neuroscience 4:1649–1677. Fukuda Y, Stone J (1974) Retinal distribution and central projections of Y–, X–, and W–cells of the cat’s retina. J Neurophysiol 37:749–772. Giolli R (1980) A review of axon collateralization in the mammalian visual system. Brain Behav Evol 17:364–398. Graybiel AM (1975) Anatomical organization of retinotectal afferents in the cat: An autoradiographic study. Brain Res 96:1–23. Graybiel AM (1976) Evidence for banding of the cat’s ipsilateral retinotectal connection. Brain Res 114:318–327. Harting JK, Guillery RW (1976) Organization of retinocollicular pathways in the cat. J Comp Neurol 166:133–144. Hendrickson A, Edwards SB (1978) The use of axonal transport for autoradiographic tracing of pathways in the central nervous system. In Neuroanatomical Research Techniques, (Robertson RT, ed) pp 242–285. New York: Academic P. Hendrickson A, Kupfer C (1976) The histogenesis of the fovea in the macaque monkey. Invest Ophthalmol 14:746–756. Hickey TL, Cox NR (1979) Cell birth in the dorsal lateral geniculate nucleus of the cat: a 3H–thymidine study. Soc Neurosci Abst 3:788. Hoffmann KP (1973) Conduction velocity in pathways from retina to superior colliculus in the cat: A correlation with receptive–field properties. J Neurophysiol 36:409–424. Hubel DH, LeVay S, Wiesel TM (1975) Mode of termination of retinotectal fibers in macaque monkey: An autoradiographic study. Brain Res 96:25–40. Hubel DH, Wiesel TN, LeVay S (1977) Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond (Biol) 278:377–409. Hughes WF, McLoon SC (1979) Ganglion cell death during normal retinal development in the chick: comparisons with cell death induced by early target field destruction. Exp Neurol 66:587–601. Illing RB (1980) Axonal bifurcation of the cat retinal ganglion cells as demonstrated by retrograde double labelling with fluorescent dyes. Neurosci Lett 19:125–130. Innocenti GM (1981) Growth and reshaping of axons in the establishment of visual callosal connections. Science 212:824–827. Itoh K, Conley M, Diamond IT (1980) Ganglion cell projections to the superior colliculus, lateral geniculate, pretectum, and vetnral lateral geniculate in the cat. Soc Neurosci Abstr 6:347. Ivy GO, Killackey HP (1981) Theontogeny of distribution of callosal projection neurons in the rat parietal cortex. J Comp Neurol 195:367–389. Jeffery G, Perry VH (1981) Evidence for ganglion cell death during development of the ipsilateral retinal projection in the rat. Dev Brain Res 2:176–180. Jeffery G, Cowey A, Kuypers HGJM (1981) Bifurcating retinal ganglion cell axons in the rat, demonstrated by retrograde double labelling. Exp Brain Res 44:34–40. Kalil R (1978) Development of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol 182:265–292. Kanaseki T, Sprague JM (1974) Anatomical organization of pretectal nuclei and tectal laminae in the cat. J Comp Neurol 158:319–338. Kelly JP, Gilbert CD (1975) The projections of different morphological types of ganglion cells in the cat retina. J Comp Neurol 163:65–80. Kliot M, Shatz CJ (!981) Siamese cat: prenatal development of the retinogeniculate pathway. Invest Ophthalmol Vis Sci Suppl 20:175. Kuwabara T, Weidman TA (1974) Development of the prenatal rat retina. Invest Ophthalmol 13:725–739. Kuypers HGJM, Catsman–Berrevoets CE, Padt RE (1977) retorgrade axonal transport of fluorescent substances in the rat's forebrain. Neurosci Lett 6:127–135. Land PW, Lund RD (1979) Development of the rat's uncrossed retinotectal pathway and its relation to plasticity studies. Science 205:698–700. Langer TL (1975) Organization of cat superior colliculus: a Golgi and degeneration study. Anat Rec 181:404. Laties AM, Sprague JM (1966) The projection of optic fibers to the visual centers in the cat. J Comp Neurol 127:35–70. LeVay S, Stryker MP (1979) The development of ocular dominance columns in the cat. In Society for Neuroscience Symposia. Vol. 4: Aspects of Developmental Neurobiology (Ferrendelli JA, ed) pp 83–98. Bethesda MD: Society for Neurosci. Lund RD, Bunt AH (1976) Prenatal development of central optic pathways in albino rats. J Comp Neurol 165:247–264. Magalhães–Castro HH, Murata LA, Magalhães–Castro B (1976) Cat retinal ganglion cells projecting to the superior colliculus as shown by the horseradish peroxidase method. Exp Brain Res 25:541–549. Mason CA (1980) Postnatal development of retinogeniculate axon arbors in thekitten. Soc Neurosci Abst 6:660. McLoon SC, Lund RD (1980) Formation and loss of a normal ipsilateral retinofugal projection in devloping chick. Soc Neurosci Abst 6:647. Mesulam MM (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non–carcinogenic blue reaction–product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26:106–117. Mize RR (1981) Morphological identification of retinal synapses in the cat superior colliculus using quantitative electron micrope autoradiography. Invest Ophthalmol Vis Sci Suppl 20:185. Northcutt RG (1980) Retinal projection s in the Australian lungfish. Brain Res 185:85–90. Northcutt RG, Przybylski RJ (1973) Retinal projections in the lamprey Petromyzon marinus (L). Anat Rec 150:400. Northcutt RG, Butler AB (1976) Retinofugal pathways in the longnose gar Lepisosteus ossous (Linnaeus). J Comp Neurol 166:1–16. Northcutt RG, Wathey JC (1980) Guitarfish possess ipsilateral as well as contralateral retinofugal projections. Neurosci Lett 20:237–242. O#146;Leary JL (1940) A structural analysis of thelateral geniculate nucleus of the cat. J Comp Neurol 73:405–430. Pollack JG, Hickey TL (1979) The distribution of retino–collicular axon terminals in rhesus monkey. J Comp Neurol 185:587–602. Rager G, Rager U (1976) Generation and degeneration of retinal ganglion cells in the chicken Exp Brain Res 25:551–553. Rakic P (1974) Neurons in the rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science 183:425–427. Rakic P (1976) Differences in the time of origin and in the eventual distribution of neurons in areas 17 and 18 of the visual cortex in rhesus monkey. Exp Brain Res Suppl 1:244–248. Rakic P (1977a) Prenatal development of the visual system in rhesus monkey. Phiols Trans R Soc Lond (Biol) 278:245–260. Rakic P (1977b) Genesis of the dorsal lateral geniculate nucleus in the rhesus monkey: site and time of origin, kinetics of proliferation, routes of migration and pattern of distribution of neurons. J Comp Neurol 176:23–52. Ramón y Cajal S (1911) Histologie du système nerveux de l’homme et d’est vertébrès. Maloine, Paris. Reprinted (1955) by Consego Superior de Investigaciones Cientificas, Madrid, Vol 2, p. 373. [Sanderson KJ, Dixon PG, Pearson LJ (1982) Postnatal development of retinal projections in the brushtailed possum, Trichosurus vulpecula. Dev Brain Res 5:161–180.] Sengelaub DR, Finlay BL (1981) early removal of one eye reduces normally occurring cell death in the remaining eye. Science 213:573–574. Shatz CJ, DiBerardino AC (1980) Prenatal development of the theretinogeniculate pathway in the cat. Soc Neurosci Abst 6:485. Sidman RL (1961) Histogenesis of mouse retina studied with thymidine–H3. In The structure of the eye (Smelser GK, ed) pp 487–506. New York: Academic P. So KF, Schneider GE (1978) Postnatal development of retinogeniculate projections in Syrian hamsters: An anterograde HRP study. Soc. Neurosci Abst 4:127. Spear PD, Smith DC, Williams LL (197) Visual receptive–field properties of single neurons in cat’s ventral lateral geniculate nucleus. J Neurophysiol 40:390–409. Sterling P (1973) Quantitative mapping with the electron microscope: retinal terminals in the superior colliculus. Brain Res 54:347–354. Stone J (1978) The number and distribution of ganglion cells in the cat’s retina. J Comp Neurol 180:753–772. Stone J, Fukuda Y (1974) A comparison of W–cells with X– and Y–cells. J Neurophysiol 37:722–748. Stone J, Rapaport DH, Williams RW, Chalupa LM (1981) Uniformity of cell distribution in the ganglion cell layer of prenatal cat retina: implications for mechanisms of retinal development. Dev Brain Res 2:231–242. Tigges J, Bos J, Tigges M (1977) An autoradiographic investigation of the subcortical visual system in chimpanzee. J Comp Neurol 172:367–380. Wässle H, Illing RB (1980) The retinal projection to the superior colliculus in the cat: a quantitative study with HRP. J Comp Neurol 190:333–356. Williams RW, Chalupa LM (1980) Projections of retinal ganglion cells in fetal cat. Soc Neurosci Abst 6:492. Williams RW, Chalupa LM (1981) Prenatal development of retinal projections to the midbrain of the cat. Invest Ophthalmol Vis Sci Suppl 20:74. [Williams RW, Chalupa LM (1984) Development of the retinal pathway to the pretectum of the cat. Neuroscience 10:1249&3150;1267.] Wilson ME, Toyne MJ (1970) Retino–tectal and cortico–tectal projections in Macaca mulatta. Brain Res 24:395–406.
Received July 27, 1981; Revised December 14, 1981; Accepted December 14, 1981
Since 11 August 98 Updated September 2, 2000
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Neurogenetics at University of Tennessee Health Science Center
| Print Friendly | Top of Page |
Mouse Brain Library | Related Sites | Complextrait.org