|
| |||||
|
| ||||||||
|
| ||||||||
Home  Publications Publications |
|
|
Development of Visual Pathways
in Mammals, pages 89–102 Note to the reader: This review summarizes the first phase of work
by Leo Chalupa and colleagues on the early development of cat retinal
projections. If you select a figure, a higher-quality image will download
into a separate window. Move this window to the side. Four years ago we initiated a research program which sought to answer two
questions: Before summarizing the progress we have made in answering these
questions, it is worth considering why we chose to study cats. First, since
the introduction of the monocular deprivation paradigm by Wiesel and Hubel
(1963), much has been learned about normal and abnormal postnatal
development of vision in cats, and this information provides a foundation
for investigating the early development of this species’ visual system.
Second, the cat is multiparous, and it is therefore possible to acquire a
sufficient number of fetuses of known gestational age. Third, the 63–65 day
gestation of the cat is long enough to allow adequate temporal resolution of
various events that occur during prenatal development. Fourth, the size of
the fetal cat’s brain is comparatively large, and thus accessible to various
types of manipulations. We began our studies by tracing the development of retinal projections to
the main visual centers, using the anterograde transport of horseradish
peroxidase and tritiated leucine. One of the tracers was injected into the
right eye, and the other tracer into the left eye, of fetuses of known
gestational age. Pregnancy was timed by exposing an estrous female to a
potent male for 24 hours. Several matings were observed during this period,
and the end of the 24 hour exposure was considered to mark the first day of
gestation (embryonic day 1, or El).
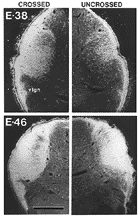
Our earliest successful injections were made on E38 (Fig. 1). At
this age virtually all ganglion cells have been generated (Walsh et al.
1983), ganglion cell growth cones have extended through the optic nerve
(Williams et al. 1983a), and at least some of the axons have reached as far
as the posterior margin of the superior colliculus (Williams,
Chalupa 1982a). As shown in figure 1, as early as E38 there is
substantial overlap of projections from right and left eyes. The
contralateral fiber influx, however, is much heavier (Fig. 1A) than
the ipsilateral (Fig. lB). Furthermore, it appears that only a
fraction of the retinal ingrowth has yet entered the terminal fields by this
age. Between E38 and E56 there is a gradual elaboration and restructuring of
retinal projections. The density of terminal label becomes much greater, and
by E46 virtually the entire lateral geniculate nucleus (Fig. 2),
pretectum (Williams, Chalupa 1983a), and superior colliculus (Williams,
Chalupa 1982a) receive heavy projections from both eyes. Overlap of
projections from right and left eyes is 100% in the geniculate nucleus (Fig.
2). Virtually complete overlap has also been described in the lateral
geniculate of another carnivore, the ferret (Linden et al. 1981), and in the
monkey (Rakic 1977). (Our findings in the cat differ, however, from those of
Shatz (1983), who reported that at all fetal ages, including E46, most of
the cat’s geniculate nucleus is innervated only by fibers from the
contralateral eye.) It is crucial to note that at E46 the pattern of
labeling is not uniform. In the geniculate nucleus the densities of the
crossed and uncrossed retinal projections appear to vary inversely. This
pattern foreshadows the development of discrete layers that contain
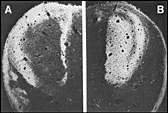
terminals of either the right or the left eye. As shown in figure 3,
by E56 segregation is essentially complete.
There are significant differences in the gestational age at which
segregation is achieved in different nuclei. Segregation starts and ends
more than a week earlier in the geniculate nucleus than in either the
superior colliculus or the pretectum (see Williams and Chalupa, 1983a).
Furthermore, the separation of retinal fibers takes place later in the C
laminae than in the A laminae of the geniculate, as was originally suggested
by Shatz (1983). Since only the A laminae receive a dominant input from the
beta class of ganglion cells, these differences may be related to the
differential maturation of the major ganglion cell types. Medium-sized
retinal ganglion cells–presumably the beta class, that project heavily to
the dorsal layers of the adult geniculate–are mostly generated before the
small cells of the gamma class, that project to the superior colliculus,
pretectum and C layers of the geniculate (Polley et al. 1981; Kliot, Shatz
1982; Walsh et al. 1983). Fig. 3. Segregation of the crossed and uncrossed retinogeniculate influx
at E56. Darkfield micrographs of peroxidase reaction product. A.
Crossed projection; B. Uncrossed projection. During gestation there is a marked overproduction of ganglion cell axons
(Ng, Stone 1982; Williams, 1983; Williams et al., 1983a,b; R.W. Williams,
M.J. Bastiani, and L.M. Chalupa, in progress). Using a quantitative electron
microscopic method, we have found that the first 100 ganglion cell axons
venture into the optic stalk on E19. Four days later, about 1,000 fibers are
present. By E28 the number of axons has increased to approximately 40,000.
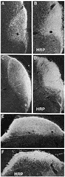
At E28 and E33 the counts include a substantial number of growth cones (Fig.
4). For instance, at E33, 3% (n = 8,000) of the neurites are growth
cones. These are characterized by extensive veil-like processes, some of
which extend radially four microns from the core (Fig. 4). Growth
cones tend to grow around the perimeter of the nerve, in apposition to glial
processes. However, some extending axons are also found in the central
region, without apparent connection to glial precursors. The peak axon count of 660,000 is attained at E39. At this age only a few
hundred growth cones were noted. A population of more than 500,000 is
maintained until E44. Between E44 and E48 there is a precipitous loss of
fibers. By E48 the axon complement has eroded to 330,000. Thereafter the
loss of axons is more gradual; the adult population of about 160,000 is not
reached until at least several weeks after birth. The excess axons produced early in development could potentially
contribute to the wide distribution of retinal projections observed during
fetal life. However, the initial massive loss of axons, which begins even
before E44, precedes by several days the onset of segregation in the
geniculate nucleus. Moreover, fiber loss is still occurring during the first
postnatal month, long after the segregation of all retinal efferents is
complete. Thus, while fiber loss may underlie the segregation of early
retinal projections, we suspect that there are other features of the
developing visual system that are shaped by this phenomenon. Early Termination of Binocular Competition Our second objective was to determine the contributions made by prenatal
binocular competition to the development of the cat’s visual pathways. For
this purpose we removed one eye from fetuses at known gestational ages
(between E40 and E56), and when these animals reached maturity we studied
the organization of their visual systems. Fig. 4. Growth cones and axons in a peripheral fasciculus of the optic
nerve at E33. To the right, dark astroblastic processes separate the
fascicles and form a limiting membrane around the nerve, the edge of which
crosses the lower left corner. Some of the very large growth cones are
marked with asterisks. Calibration bar is 1 µm.
Download a high-resolution 300 KB image. In these enucleated animals, the remaining retina innervates the entire
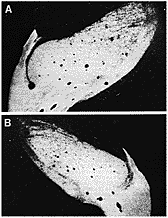
ipsilateral and contralateral geniculate nuclei (Fig. 5) (Williams,
Chalupa 1982b & 1983b; Williams 1983). However, as shown in figure 5B,
the geniculate ipsilateral to the remaining eye is substantially smaller
than that on the contralateral side. The morphology of the lateral
geniculate nuclei of these one-eyed cats is characterized by two laminae: a
dorsal magnocellular layer and a ventral layer. A similar result has been
described by Rakic (1981) in prenatally enucleated monkeys. Presumably, the
magnocellular layer corresponds to what would normally have been layers A
and Al, whereas the ventral layer corresponds to what would have been
subdivided into layers C, C1, C2, and C3. Thus, the six laminae of the
normal cat’s geniculate are supplanted by two composite layers. We have also shown that there are significantly more optic nerve fibers
in prenatally enucleated cats than is normal (Williams et al. 1983b).
Furthermore, the number of fibers within the optic nerve of these animals
matches the number of ganglion cells in the remaining retina (Henderson et
al. 1983; Chalupa et al., in review). The results of these experiments,
summarized in table 1, show that the prenatally enucleated cats have
about 20% or 30,000 more ganglion cells than normal animals. Obviously this
excess could contribute to the widespread retinal projections found in
one-eyed cats. An unexpected result of this study was that the number of ganglion cells
saved does not depend critically upon the gestational age at which
enucleations are performed. Removal of an eye at E42, when there are more
than 500,000 fibers in the optic nerve, is no more effective in saving
ganglion cells from imminent death than is enucleation at E51, when there
are 300,000 axons. Fig. 5. Retinogeniculate projections in an adult cat from which one eye
was removed on E49. The crossed projection is shown in A; the uncrossed in
B. parts of both nuclei are labeled with the peroxidase chromogen,
however, the ipsilateral nucleus and the ipsilateral retinal projection are
considerably smaller. *All animals were mature Early eye removal has been shown to maintain widespread retinal
projections in a number of mammalian species (e.g., Chow et al. 1973;
Sanderson 1978; Land, Lund 1979; Frost, Schneider 1979 Rakic 1981); however,
relatively little is know about the functional organization of the altered
retinal connections (but see Rhoades, Chalupa 1980). Accordingly, we sought
to determine the functional properties of the retinal influx to the
geniculate of prenatally enucleated cats. For this purpose, extracellular
single cell recordings were made within the lateral geniculate,
contralateral and ipsilateral to the remaining eye of adult animals from
which an eye had been removed more than two weeks before birth. The results
are clear-cut: all regions of the geniculate nuclei of these cats are
functionally innervated by the retina input, and the topographic
organization is similar to that of the normal cat (Williams, Chalupa 1982 &
1983b; Williams 1983). Furthermore, injection of peroxidase into the medial
region of the geniculate that contains the area centralis representation
revealed a normal decussation pattern in the remaining retina (Williams,
Chalupa 1983b). This result rules out the possibility that an aberrant
retinal input from the inappropriate hemiretina was either functionally
suppressed or ineffective in driving thalamic units. Parenthetically, it
should be noted, that we have found recently a clear decussation pattern
following unilateral injection of HRP as early as E44 (Lia et al., 1983). Even though the functional organization of the lateral geniculate nucleus
appeared normal in prenatally enucleated cats, it seemed important to also
examine the properties of the visual cortex in these animals. Studies of the
visual cortex were carried out in collaboration with Drs. Brenda Shook and
Lamberto Maffei (B.L. Shook, L. Maffei, and L.M. Chalupa, 1983). Small
iontophoretic deposits of horseradish peroxidase conjugated to wheat germ
agglutinin were made into the A lamina of normal cats and into the most
dorsal portion of the geniculate nuclei of enucleated animals. As expected,
discrete patches of label were found in layer IV of normal cats, whereas
continuous label was found in the enucleated cats. Thus, in agreement with
the previous work of Rakic (1981), ocular dominance columns fail to develop
following early removal of one eye. In the same cats long tangential microelectrode penetrations were made
through area 17. Most of these penetrations extended 2.5–3.0 mm at an
oblique angle down the medial bank of the marginal gyrus, and thus the
electrode traversed a region that in normal cats is occupied by a number of
discrete ocular dominance columns. In the prenatally enucleated cats, the
remaining eye could activate all neurons, and the activity did not show any
signs of waxing and waning. In contrast, in animals enucleated as adults,
regions of reduced visual activity were recorded when similar penetrations
were made. It is particularly noteworthy that cortical neurons of prenatally
enucleated animals exhibited an orderly progression in orientation
selectivity. In several penetrations, sequences of cells were encountered
that had a 180 degree cycle of preferred orientations. This is
characteristic of hypercolumns in the visual cortex of normal animals (Hubel,
Wiesel 1962). Therefore, this finding indicates that orientation columns can
develop independently of ocular dominance columns. There was one significant difference in the visual receptive field
properties between prenatally enucleated and normal cats. Within 50 of the
area centralis representation–where most penetrations were confined–the
dimensions of receptive fields were significantly smaller in prenatally
enucleated animals than in normal cats. One possible explanation for this
intriguing finding is that dendritic fields of ganglion cell may be smaller
than normal. We know that the remaining retina of these animals has about
30,000 more ganglion cells than normal, yet the retinal area is not
appreciably larger. One would anticipate that exacerbated dendro-dendritic
competition among ganglion cells in the remaining retina (see Perry, Linden
1982) would result in a reduction in the size of dendritic fields. Such a
reduction would permit accommodation of the excess ganglion cell complement
without disrupting the retinal mosaic. ACKNOWLEDGEMENT: Supported by grants EY03391 and EY05670 from the
National Eye Institute of NIH. REFERENCES Chalupa LM, Williams RW, Henderson Z. Binocular competition in the fetal
cat regulates the size of the ganglion cell population. Neuroscience
12:1139–1146. Chow EL, Mathers LH, Spear PD (1973) Spreading of uncrossed retinal
projection in superior colliculus of neonatally enucleated rabbits. J Comp
Neurol 150:307. Frost DO, Schneider GE (1979) Plasticity of retinofugal projections after
partial lesions of the retina in newborn Syrian hamsters. J Comp Neurol
185:517 Henderson Z, Williams RW, Chalupa LM (1983) Removal on an eye in the
fetal cat increases the number of ganglion cells maintained in the remaining
retina. Invest Ophthalmol Vis Sci 24:290. Hubel DH, Wiesel TN (1962) Receptive field, binocular interaction, and
functional architecture in the cat’s visual cortex. J Physiol (Lond)
160:106. Kliot M, Shatz CJ (1982) Genesis of different retinal ganglion cell types
in the cat. Soc Neurosci Abst 8:815. Land PW, Lund RD (1979) Development of the rat’s uncrossed retinotectal
pathway and its relation to plasticity studies. Science 205:698. Lia B, Williams RW, Chalupa LM (1983). Early development of retinal
specialization: the distribution and decussation patterns of ganglion cells
in the prenatal cat demonstrated by retrograde peroxidase labelling. Soc
Neurosci Abstr 9:702. Linden DC, Guillery RW, Cucchiaro J (1981) The dorsal lateral geniculate
nucleus of the normal ferret and its postnatal development. J Comp Neurol
203:189. Ng AYK, Stone J (1982) The optic nerve of the cat: Appearance and loss of
axons during normal development. Develop Brain Res 5:263. Perry VH, Linden R (1982) Evidence for dendritic competition in the
developing retina. Nature 297:683. Polley EH, Walsh C, Hickey TL (1981) Neurogenesis in cat retina: A study
using 3H-thymidine autoradiography. Soc Neurosci Abstr 7:672. Rakic P (1977) Prenatal development of the visual system in rhesus
monkey. Phil Trans R Soc Lond B 278:245. Rakic P (1981) Development of visual centers in the primate brain depends
on binocular competition before birth. Science 214:928. Rhoades RW, Chalupa LM (1980) Effects of neonatal enucleation on
receptive-field properties of visual neurons in superior colliculus of the
golden hamster. J Neurophysiol 43:595. Sanderson KJ, Pearson LJ, Dixon PG (1978) Altered retinal projections in
brushtailed possum, Trichosurus vupecula, following removal of one
eye. J Comp Neurol 143:101. Shatz CJ (1983) The prenatal development of the cat’s retinogeniculate
pathway. J Neurosci 3:482. Shook BL, Maffei L, Chalupa LM (1983) Functional organization of the
cat’s visual cortex after prenatal unilateral enucleation. Soc Neurosci Abst
9: in press. Walsh C, Polley EH, Hickey TL, Guillery RW (1983) Generation of cat
retinal ganglion cells in relation to central pathways. Nature 302:611. Wiesel TN, Hubel DH (1963) Single-cell responses in striate cortex of
kittens deprived of vision in one eye. J Neurophysiol 26:1003. Williams RW, Chalupa LM (1982a) Prenatal development of retinocollicular
projections in the cat: An anterograde tracer transport study.
J Neurosci 2:604. Williams RW, Chalupa LM (1982b) The effects of prenatal unilateral
enucleation upon the functional organization of the cat’s lateral geniculate
nucleus. The Physiologist 25:223. Williams RW (1983) Prenatal Development of the Cat’s Visual System.
Thesis, University of California, Davis. Williams RW, Chalupa LM (1983a) Development of the retinal pathway to the
pretectum of the cat. Neuroscience, in press. Williams RW, Chalupa LM (1983b) Expanded retinogeniculate projections in
the cat following prenatal unilateral enucleation: Functional and anatomical
analyses of an anomalous input. Soc Neurosci Abstr 9: in press. Williams RW, Bastiani MJ, Chalupa LM (1983a) Addition and attrition of
axons within the optic nerve during fetal development: Appearance of growth
cones and necrotic axons. Invest Ophthalmol Vis Sci Suppl 24:8. Williams RW, Bastiani MJ, Chalupa LM (1983b) Loss of axons in the cat
optic nerve following fetal unilateral enucleation: An electron microscopic
analysis. J Neurosci 3:133.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Neurogenetics at University of Tennessee Health Science Center
| Top of Page |
Mouse Brain Library | Related Sites | Complextrait.org