|
| ||||||
|
| ||||||||
|
| ||||||||
Home  Publications Publications |
|
|
Genetic Analysis of Variation in Neuron Number Richelle Cutler Strom Chapter 5: Mapping Genes Controlling Variation in Retinal Ganglion Cell Number Using Recombinant Inbred Strains and F2 Intercross Progeny The high heritability of retinal ganglion cell number among mouse strains indicates that the variation in ganglion cell number results primarily from genetic differences. Furthermore, the prominent bimodality in ganglion cell number among 57 inbred strain averages demonstrates that only a few genes with major effects are responsible for variation in ganglion cell number. Together, these findings suggest that it should be possible to map QTLs that are responsible for variation in ganglion cell number between strains. Only one other study has mapped QTLs controlling neuron number in mice. This study, by Dains et al. (1994), examined variation in the number of striatal cholinergic neurons among inbred mouse strains. A greater than 50% variation was found between BXD strains. The narrow-sense heritability in the BXD strains was found to be around 0.30 and the number of effective factors was from 3 to 4. Using BXD strains, QTLs associated with variation in cholinergic neuron number were mapped to chromosomes 1, 6, 9, 11 and 12. In this study, I use BXD recombinant inbred strains and two F2 intercrosses in a linkage analysis to map QTLs that produce variation in retinal ganglion cell number. In order to map genes that modulate ganglion cell number directly, rather than genes that modulate global neuron number, I have mapped using ganglion cell number residuals regressed with respect to brain weight. Thus, the QTLs that are detected are expected to have specific effects on the ganglion cell population. Variation in neuron number After detecting significant linkage between a QTL interval and variation in ganglion cell number, the next step is to look for candidate genes within the QTL interval. There are two criteria for selecting candidate genes that map within the QTL interval. The first is that it is expressed during prenatal or early postnatal eye development, and the second is that it has an assigned function consistent with a role in cell production or cell death. In this study, I examine two candidate genes that meet these criteria. The candidate genes were examined by comparing ganglion cell numbers in wild type mice to those in mice carrying a nonfunctional transgene at the candidate locus. The BXD recombinant inbred strains were used to map genes modulating ganglion cell number. Two F2 intercrosses were then used to confirm QTLs mapped in the BXD RI strains and to map additional QTLs. I generated two groups of intercross progeny from matings between inbred strains BALB/cJ and CAST/Ei (CCASF2, n =112), and BXD32 and CAST/Ei (32CASF2, n =140). The parental strains BALB/cJ, BXD32 and CAST/Ei have ganglion cell numbers of 63,400, 75,800, and 45,000, respectively. To perform a genome-wide linkage analysis DNA was genotyped from CCASF2 and 32CASF2 individuals at 110 microsatellites. Candidate genes were tested by comparing ganglion cell counts from mice containing a nonfunctional transgene at the candidate locus to ganglion cell counts from mice carrying a normal functional candidate. Two candidate genes were tested, retinoic acid receptor alpha 1 (Rara1) and thyroid receptor alpha (Thra). The Rara1 null mice were obtained from Vincent Giguere at Royal Victoria Hospital in Montreal, Canada, while the Thra null mice were obtained from Douglas Forrest at Mount Sinai in New York. The homozygous Rara1 null mice appeared completely normal, whereas the Rara null mice had high postnatal lethality and testis degeneration (Lufkin et al., 1993). The homozygous Thra null mice have a 20% lower heart rate and a 0.5 °C lower body temperature, but otherwise are healthy and fertile (Wikstrom et al., 1998). The examination of the Rara1 and Thra knock-out mice described here is preliminary work carried out as part of a collaboration with Drs. Vincent Giguere and Douglas Forrest. The preliminary work on Rara1 has been published (Zhou et al. 1998). Final publications are in progress (Zhou G, Strom R, Giguere V, Williams RW). The average ganglion cell number in the BXD strains ranges from 52,500 in BXD27 to 76,200 in BXD32. There are 8 strains with low ganglion cell means and 14 strains with high ganglion cell means. In Table 5.1, the low strains are assigned L and the high strains are assigned H. There are five strains with intermediate ganglion cell numbers that fall within ± 2,000 of the mid-parental value of 59,000 and these strains are assigned an I in Table 5.1.
Table 5.1 Corrected retinal ganglion cell number for BXD strains and parents, C57BL/6J and DBA/2J.
A comparison of ganglion cell number in the 26 BXD strains and the genotypic strain distribution at 529 loci resulted in the best match at Tstap91A located on Chr 11 at 75 cM. A correlation between alleles at Tstap91A and ganglion cell number resulted in a coefficient of +0.72. The LOD score for the linkage between ganglion cell number and Tstap91A is 4.5. Table 5.2 shows the concordance between the genotypic strain distribution of loci on Chr 11 and the putative genotype at the RGC locus based on its parental resemblance. There are 20 strains with RGC phenotypes concordant with alleles at Tstap91A, while only 1 strain is discordant and 5 strains are intermediate.
Table 5.2. BXD strain distribution pattern for retinal ganglion cell number and genotypes at five loci on Chr 11.
* The alleles for RGC number are based on the strain’s resemblance to the C57Bl/6J (B) or DBA/2J (D) phenotype. The B allele was assigned if ganglion cell number was <57,000 and the D allele was assigned if the ganglion cell number was >61,000. Strains with ganglion cell numbers >57,000 but <61,000 were assigned an intermediate (I) allele type.
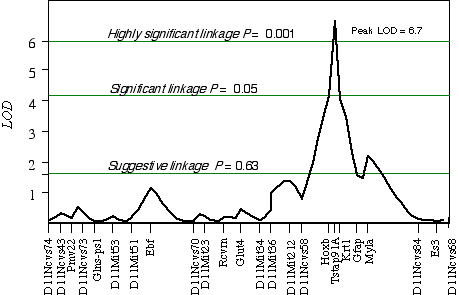
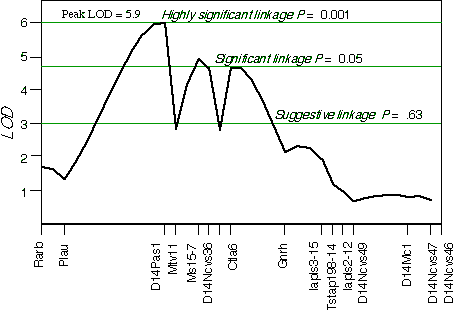
Controlling for the Tstap91A indicated secondary loci on distal Chr 1 at D1Ncvs60 and on proximal Chr 7 at Iapls3-4;1-11. Controlling for these two secondary loci increased the LOD of Tstap91A to 6.7 at 57 cM, with a 2 LOD CI between Hoxb and Krt1, at 56 and 58 cM, respectively (Fig 5.1). The second close match with ganglion cell number was found at D14Pas1 on Chr 14 at 12 cM, with a correlation coefficient of +0.60 and an LOD score of 3.5. A linkage test while controlling for the variance at D14Pas1 detected secondary loci on Chr 2 at D2Byu2 and on Chr 6 at D6Mit86. Interval mapping while controlling for these two secondary loci increased the LOD at D14Pas1 to 5.9 at 15 cM, with a 2 LOD confidence interval from 11 to 17 cM (Fig 5.2). A permutation test in the BXD data set while controlling for secondary loci defined the appropriate genome-wide conditional significance thresholds. The LOD scores required for a suggestive, significant and highly significant linkage are 3.0, 4.7, and 6.0, respectively. The QTLs detected on Chr 11 with peak LOD score of 6.7 exceeds the highly significant threshold. The locus on proximal Chr 14 with a peak LOD score of 5.9, almost reaches the highly significant threshold. The QTL mapped to Chr 11 has been named Neuron number control 1 (Nnc1) (Williams et al., 1998), and the QTL mapped to Chr 14 has been named Neuron number control 2 (Nnc2).
Figure 5.1. Linkage map demonstrates the QTL Nnc1 on Chr 11 in the BXD data set. LOD scores were computed at 1 cM intervals while controlling for Iapls3-4; 1-11 on Chr 7 and D1Ncvs60 on Chr 1.
My analysis detected a significant linkage on Chr 14 that was not reported in Williams et al., (1998). A possible explanation for the additional linkage is a difference in the regression procedure. In this proceeding, I truncated the ganglion cell numbers in the BXD5 and BXD32 strains before the regression of ganglion cell number and brain weight. I did this because the ganglion cell numbers for BXD5 and BXD32 exhibited overdominance and in the case of BXD5 also an overdominance in brain weight. In a regression analysis, a large deviation in just a few data points can distort the true association. The overdominance in these strains may result from unique non-additive epistatic interactions or a combination of genes with increaser alleles, which could produce a global increase in cell number, and may interfere with linkage associations.
Figure 5.2.Linkage map demonstrates the QTL Nnc2 on Chr 14 in the BXD data set. LOD scores were computed at 1 cM intervals while controlling for D2Byu2 on Chr 2 and D6Mit86 on Chr 6.
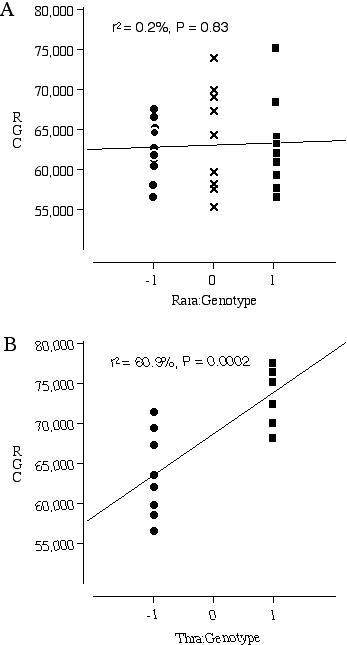
Testing Nnc1 candidate genes by examining knock out mice Three excellent candidates for Nnc1 that map within the Hoxb—Krt1 interval, retinoic acid receptor alpha 1 (Rara1), thyroid receptor alpha (Thra), and the neuregulin receptor Erbb2. All three of these receptors are expressed in the developing retina and the ligands for these receptors are known to influence retinal cell number (Bermingham-McDonogh et al., 1996; Ruberte et al., 1993; Sjoberg et al., 1992). The addition of neuregulin, the ligand that activates the receptor Erbb2, promotes ganglion cell survival in culture (Bermingham-McDonogh et al., 1996). The addition of retinoic acid and thyroid hormone to retinal cultures increases the differentiation of photoreceptors (Kelley et al., 1995). A reciprocal relationship in differentiation potential is thought to occur between the early- and late-generated retinal cell types. Thus, an increased bias for the generation of ganglion cells could have repercussions on the number later-generated photoreceptors. The average ganglion cell number in the transgenic mice carrying a null Rara1 is 62,494 ± 1160 (n = 10) compared to the average for normal Rara1 mice of 62,998 ± 1930 (n = 9) (Fig. 5.3A). There are no significant differences in ganglion cell number between the homozygous mutant Rara1 and the normal Rara1 mouse, p = 0.82. In contrast, the average number of ganglion cells in the transgenic mice carrying a null Thra1 is 63,645 ± 1884 (n = 9), compared to an average of 74,030 ± 1137 (n = 8) in mice carrying a normal Thra1 (Fig. 5.3B). The difference in ganglion cell number between the homozygous null and normal Thra mice is highly significant at p = 0.0005.
Figure 5.3. Ganglion cell numbers for transgenic mice carrying homozygous null Nnc1 candidate genes (–1), heterozygous null (0) and wildtype genes (1). (A) Rara1 (–/–)(–/+)(+/+) comparison; (B) Thra (–/–)(+/+) comparison.
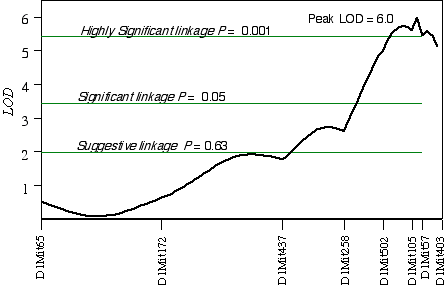
In CCASF2 mice, ganglion cell number corrected with respect to brain weight ranged from 41,000 to 70,000. I performed a linkage analysis between ganglion cell number and the genotypes of 30 extreme CCASF2 progeny. The best association with ganglion cell number was on distal Chr 1 at D1Mit105 with a LOD score of 3.5. The second best linkages were on proximal Chr 15 at D15Mit12 with a LOD score of 3.2 and on mid-distal Chr 7 at D7Mit238 with a LOD score of 2.3. Linkage testing while controlling for the Chr 1 locus detected a linkage on Chr 16 near D16Mit29. Composite interval mapping on Chr 1 while controlling for the QTL intervals on Chrs 7, 15, and 16 resulted in an LOD score of 9.3 between D1Mit502 and D1Mit105. Linkage testing while controlling for D15Mit12 detected a linkage on Chr 10 near D10Mit282 with a LOD score of 2.7. Interval mapping while controlling for the QTLs on Chrs 1, 7, and 10 increased the LOD at D15Mit12 to 4.0. Increasing the number of progeny in a linkage analysis can improve the estimate of the QTL location. Interval mapping on Chr 1 with 100 CCASF2 while controlling for D15Mit12 and D13Mit18 resulted in an LOD of 6.0 between D1Mit105 and D1Mit57, with the peak score at 82 cM, 2 cM distal of D1Mit105 (Fig. 5.4). The 2 LOD CI is from 77 cM to 88 cM. Variation at D1Mit105 is estimated to explain 15% of variance in cell number, however, estimates in small sample sizes such as this one, tend to overestimate the effect size. Interval mapping on Chr 15 with 100 CCASF2 and controlling for D1Mit105 resulted in an LOD of 2.0 at D15Mit12, the most proximal marker. A permutation test with the complete data set while controlling for secondary loci defined the genome-wide significance thresholds. The LOD scores required for a suggestive, significant and highly significant linkage are 2.0, 3.5, and 5.4, respectively. The LOD score reached 2.0 at D15Mit12 and is therefore only a suggestive linkage. The LOD score in the interval between D1Mit105 and D1Mit57 reaches 6.0, and is a highly significant linkage. I have named the QTL on distal Chr 1 neuron number control 3 (Nnc3).
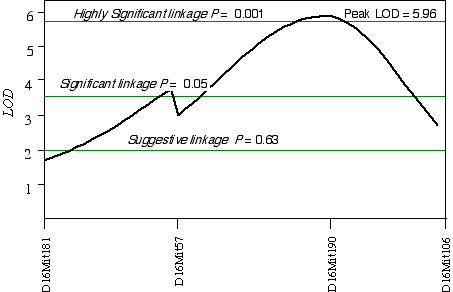
In 32CASF2 mice, ganglion cell number corrected with respect to brain weight ranged from 41,000 to 70,000. A comparison between ganglion cell number and genotypes in the 45 extreme 32CASF2 progeny detected a linkage on mid-Chr 16 at D16Mit190 with a LOD score of 2.6. A linkage test while controlling for the D16Mit190 resulted in detecting secondary loci D13Mit203 and D17Mit10. Composite interval mapping on Chr 16 while controlling for D13Mit203 and D17Mit10 increased the LOD score to 6.0 at D16Mit190, which maps at 41.5 cM (Fig. 5.5). The 2 LOD CI is from 22 cM to 50 cM. A permutation test defined the appropriate genome-wide significance thresholds. The LOD scores required for a suggestive, significant and highly significant linkage are 2.0, 3.6, and 5.9, respectively. The linkage at D16Mit190 with a LOD score of 6.0 is therefore a highly significant linkage. I have named the QTL on Chr 16 neuron number control 4 (Nnc4).
Figure 5.5. Linkage map demonstrates Nnc4 on Chr 16 in the 32CASTF2 data set. LOD scores were computed at 1 cM intervals while controlling for D13Mit203 and D17Mit10.
Discussion Synopsis I have mapped four significant QTLs that modulate ganglion cell number in mice to Chr 1, 11, 14, and 16. These QTLs have been named Nnc1, 2, 3, and 4. Nnc1 maps to 57 cM on Chr 11, Nnc2 maps to 15 cM on Chr 14, Nnc3 maps to 82 cM on Chr 1, and Nnc4 maps to 41.5 cM on Chr 16. Candidates The significantly reduced ganglion cell number in mice carrying a null Thra transgene compared to the wildtype supports the candidate gene Thra for Nnc1. Thra maps within 1–2 cM of Nnc1 on chromosome 11 (Montgomery et al., 1997), and is expressed within the developing chick retina (Sjoberg et al., 1992). The ligand of Thra, triiodothyronine, is known to influence retinal ganglion cell fate determination (Hoskins, 1985), retinal maturation rate (Macaione et al., 1984). Hypothyroidism during retinal development results in decreased cell density in the ganglion cell layer (Hoskins, 1985; Navagantes et al., 1996). Interestingly, the addition of the Thra ligand, triiodothyronine, to cultured fetal rat hypothalamus cells stimulates the release IGF-1, a known mitogen in retinal cultures (Binoux et al., 1985). A candidate gene for Nnc2 would map on Chr 14 between 11 cM and 17 cM, preferably near the peak linkage statistic at 15 cM. Two fantastic candidates for Nnc2 are bone morphogenic protein receptor–2/4 (BMPR) and bone morphogenic protein (BMP). BMPR and BMP map to 13 cM and 14 cM (Beppu et al., 1997). BMPR–2 is a serine–threonine kinase receptor that is strongly expressed in the developing optic vesicle of the rat (Obata et al., 1999), and in differentiating ganglion cells in the chick (Carri et al., 1998). The function of BMPR in the neural retina is not known, however, in the brain BMPRs are involved in proliferation and differentiation (Zhang et al., 1998). The candidate genes for Nnc3 would map on Chr 1 between 77 cM and 88 cM, preferably close to the peak linkage statistic at 82 cM. The best Nnc3 candidate is retinoid X receptor gamma (Rxrg). Rxrg maps just within the QTL interval at 88 cM and is expressed in the developing mouse retina (Dolle et al., 1994). Rxrg null mice appear normal with respect to growth, behavior, fertility and viability (Krezel et al., 1996). Interestingly, Rxrg forms a heterodimer with Thra, the Nnc1 candidate, and together the pair binds to thyroid hormone response elements to activate gene transcription (Force et al., 1994). The candidate genes for Nnc4 would map on Chr 16 between 22 cM and 50 cM with the peak association at 41.5 cM. The 2 LOD confidence interval for this QTL interval is very broad and searching for a candidate gene within this broad an interval is essentially an exercise in optimism. However, an attractive candidate does map within the interval at 27 cM, and this gene is the enhancer of split homolog–1 (HES1). HES1 is basic helix-loop-helix (bHLH) transcription factor expressed in progenitor cells of the developing neural retina (Kageyama et al., 1997). Interestingly, HES1 is involved in the Notch pathway, where it acts downstream of Notch to inhibit neural differentiation. In mice carrying a null HES1 transgene the retina differentiates prematurely and forms abnormal rosette structures (Tomita et al., 1996). Genetic variants in HES1 could alter the expression level or timing of HES1 and this would affect the number of retinal progenitors produced.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Neurogenetics at University of Tennessee Health Science Center
| Text Only | Top of Page |
Mouse Brain Library | Related Sites | Complextrait.org