Note to the reader: This is a revised edition of a paper published in the Journal of Cognitive Neuroscience (2000;12828–839). The definitive original print version is copyright ©2001 by The Massachusetts Institute of Technology.
New figures, text, and links have been incorporated into the revision. Revised html (http://nervenet.org/netpapers/Rosen/Startle2000/Startle.html) copyright ©2001 by Glenn
D. Rosen
| Click here for a .pdf (250K) copy of the paper |
IMPAIRED PROCESSING OF COMPLEX AUDITORY STIMULI IN RATS WITH INDUCED CEREBROCORTICAL MICROGYRIA: AN ANIMAL MODEL OF DEVELOPMENTAL LANGUAGE DISABILITIES
Matthew G. Clark,1 Glenn D. Rosen,2 Paula Tallal,1 and R. Holly Fitch3
1Center for Molecular and Behavioral Neuroscience, Rutgers University, Newark, NJ;2Dyslexia Research Laboratory, Division of Behavioral Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School , Boston, MA; 3 Biobehavioral Sciences Graduate Degree Program, University of Connecticut, Storrs, CT.
Address all correspondence to:
R. Holly Fitch, Ph.D.
Biobehavioral Sciences Graduate Degree Program
University of Connecticut
3107 Horse Barn Hill Rd
U-154
Storrs, CT 06269
EMAIL: hfitch@psych.psy.uconn.edu
Individuals with developmental language disabilities, including developmental dyslexia and specific language impairment (SLI), exhibit impairments in processing rapidly presented auditory stimuli. It has been hypothesized that these deficits are associated with concurrent deficits in speech perception and, in turn, impaired language development. Additionally, post mortem analyses of human dyslexic brains have revealed the presence of focal neocortical malformations such as cerebrocortical microgyria. In an initial study bridging these research domains, we found that male rats with induced microgyria were impaired in discriminating rapidly presented auditory stimuli. In order to further assess this anatomical-behavioral association, we designed two experiments using auditory reflex modification. These studies were intended to assess whether auditory processing deficits in microgyric male rats would be seen in threshold detection of a silent gap in white noise, and in oddball detection of a two-tone stimulus of variable duration. Results showed no differences between sham and microgyric subjects on gap detection, but did show that microgyric subjects were impaired in the discrimination of two-tone stimuli presented in an oddball paradigm. This impairment was evident for stimuli with total durations of 64 msec or less, while both groups were able to discriminate stimuli with durations of 89 msec or greater. The current results further support the relationship between malformations of the cerebral cortex and deficits in rapid auditory processing. They also suggest that the parameters characterizing rapid auditory processing deficits for a specific task may be influenced by stimulus features and/or cognitive demand of that particular task
Developmental language learning disabilities, such as developmental dyslexia (specific reading disability) and specific language impairment (SLI), are characterized by a significant limitation in reading and/or language development and ability without the presence of an overt underlying condition such as low overall IQ or impaired hearing (1). Moreover, individuals with developmental language disabilities typically exhibit deficits in speech perception and, more specifically, processing of phonemes incorporating rapid change (e.g., stop consonants; 2–6). Interestingly, this processing impairment has been observed for non-linguistic stimuli as well. For example, Tallal and Piercy (7,8) demonstrated that normal children were able to discriminate two 75 msec tones separated by an inter-stimulus interval (ISI) as short as 8 msec, while individuals with SLI required an ISI exceeding 300 msec to perform the same discrimination at the same level of accuracy. Similar rate-specific auditory processing deficits have been observed in dyslexics behavior and neurophysiology, using both speech and non-speech stimuli (5,9–19). These accumulated findings (see also 1,20,21 for reviews) overwhelmingly support the view that individuals with developmental language disabilities have a fundamental dysfunction in the ability to process brief auditory stimuli followed in rapid succession by other acoustic information (i.e., rapid auditory processing). Indeed, in a review of studies on SLI, Leonard (1) writes: “Among the most enduring findings in the literature on SLI is the finding that children with SLI perform quite poorly on tasks requiring the processing of brief stimuli and the processing of stimuli that are presented in rapid succession.”
These findings have been assimilated into a theoretical framework advanced by Tallal and colleagues (see 22). This model predicts that an impaired ability to process and discriminate rapidly changing auditory information will lead to severe impairments in speech perception, particularly for phonemic signals that incorporate rapid change (i.e., formant transitions). This causal association is supported by evidence that non-lingual auditory processing thresholds in infants and toddlers predict significantly to later language outcome (23,24). Such a bottom-up model of speech and language development also predicts that speech perception deficits will exert cascading developmental effects on phonological representation and phonological-orthographic association (i.e., reading acquisition), a notion supported by evidence that more than 80% of SLI children go on to develop reading impairments (25,26). This model forms one framework within which we can characterize the association between focal cortical malformations as seen in dyslexic brains, and auditory processing deficits as seen in language disabled populations, using an animal model.
Interestingly, individuals with developmental language disabilities do not show equivalent deficits on all rapid auditory processing tasks. For instance, no group difference in gap detection threshold was found for adults with developmental dyslexia as compared to control adults (27,28). Conversely, infant gap detection thresholds do predict significantly to later language performance in toddlers (29,30), and gap detection thresholds appear to be significantly higher for SLI and reading disabled children as compared to control children (e.g., 31,32). Since gap detection tasks are generally accepted as a means to assess temporal auditory acuity (33), these conflicting results suggest that temporally-dependent auditory deficits associated with developmental dyslexia and SLI may interact with the stimulus characteristics of a specific task, as well as task difficulty or demand (which in turn may be age-dependent). Clearly, further characterization of task and stimulus parameters which elicit processing deficits might help in pinpointing the neurobiological basis for these deficits, as well as providing neurobiological insight into the top-level behavioral profile comprising language disability.
Concurrent basic research on the neural substrate of language disabilities has also progressed from the perspective of neuroanatomical characterization of affected individuals. Specifically, Galaburda and colleagues (34–36) reported that postmortem analyses on the brains of dyslexics revealed developmental neuropathologic anomalies including cerebrocortical microgyria, glial scars, dysplasias and ectopic collections of neurons in the molecular layer of neocortex. Although these focal developmental anomalies were present bilaterally throughout the cortex, they were found predominantly in the left perisylvian and inferior prefrontal regions of neocortex. Subsequently, animal models exhibiting virtually identical malformations to those seen in dyslexic brains have been developed. For example, rat models of focal cortical microgyria induced via postnatal day 1 (P1) focal freezing lesions have been reliably generated (37,38; see Figure 1).
Recently, we utilized a microgyric rat model to relate the above behavioral and neuroanatomical features of language disorder (39). Specifically, we demonstrated that adult male rats with induced bilateral microgyria in primary somatosensory (SM-I), occipital or frontal cortices were impaired in rapid auditory discrimination when compared to unlesioned shams (39–42). In these studies, we used a task modeled on Tallal’s original two-tone sequence discrimination test for children with SLI (see 7). Rats were shaped over a period of months to perform a go/no go, two-tone target identification of stimuli with total stimulus durations of 540 msec, 390 msec, 332 msec, or 249 msec. Total stimulus duration was reduced from 540 to 249 msec over a period of 24 days of testing (6 days at each of 4 conditions). Results showed that all rats were able to discriminate at the longer stimulus durations, but at the 249 msec (“fast”) condition, the microgyric subjects were significantly impaired compared to sham subjects, regardless of lesion location.
These results provided a critical new connection between previously disparate fields addressing the behavioral and neuromorphological features of language disability. Specifically, we had evidence that the cortical developmental malformations seen in dyslexic brains are clearly associated with auditory processing deficits seen in language disabled populations. Yet, the modified operant conditioning paradigm represents an enormously time-consuming assessment method, and performance on this task is potentially confounded by learning and/or motivation. Hence we endeavored to adapt an auditory processing paradigm which could reduce testing/training time and eliminate these confounds, and allow for further characterization of auditory processing deficits in our animal model.
The paradigm we adapted to this purpose is reflex modification, also called startle reduction. In reflex modification, subjects do not require training, motivation or dietary manipulation. Moreover, testing is accomplished far more quickly than the operant task (i.e., weeks for the reflex modification testing versus months for the operant task), allowing for more trials at each condition and thus, a more precise characterization of threshold differences between microgyric and sham groups. The reflex modification paradigm (see 43,44 for review) consists of the presentation of a benign stimulus (pre-pulse stimulus, or pre-stimulus) briefly preceding the presentation of a startle-eliciting stimulus (SES). When the pre-stimulus is detected, the amplitude of the startle reflex (including the whole body acoustic startle reflex of the rat or the eye-blink reflex of humans) is inhibited. This phenomenon is called pre-pulse inhibition. The extent of pre-pulse inhibition is related to the overall detectability of the pre-stimulus. Comparison of reflex amplitudes when a pre-stimulus is present (i.e., a cued trial) versus not present (i.e., an uncued trial) provides an objective measure of sensory detection (cf. 45)
Using this method, we designed two experiments to assess whether the deficit in rapid auditory processing in microgyric rats could be elicited in a paradigm with a simple temporal demand (as measured by threshold for detection of a silent gap in broadband white noise; Experiment 1), as well as a paradigm measuring oddball detection of a variable duration two-tone stimulus (Experiment 2)
A simple and well-accepted method of measuring the temporal resolution within the auditory domain is gap detection (33). Many gap detection tasks measure the ability to detect brief gaps of silence in an otherwise continuous background broadband white noise. Plomp (46) suggested the rate of neural decay is gradual following noise offset. From this model, the least detectable gap is the minimum duration following noise offset wherein neural activity has decayed to a level sufficient to be detected, given the subsequent increase in neural activity at noise onset. Since auditory gap detection tasks of this sort typically employ broadband white noise presented binaurally, variables such as spectral (frequency) and spatial processing (sound localization) are held constant. Thus, the task measures threshold parameters of a single dimension—processing of temporal information in the central auditory system.
Since evidence suggests that auditory processing deficits associated with developmental language disabilities are temporally dependent (22), we adapted a gap detection task to a reflex modification paradigm (Experiment 1; Figure 2). In this paradigm, silent gaps of variable duration, embedded in continuous low-level background white noise, served as the pre-stimulus cues. On a given trial, subjects were provided with a pre-stimulus gap varying in duration from 0 to 50 msec (with 0 msec gap trials representing the baseline or uncued condition). Reflex amplitudes were then compared across trials as a function of gap duration, with the minimum gap to produce a significant attenuation of reflex response representing the detection threshold.
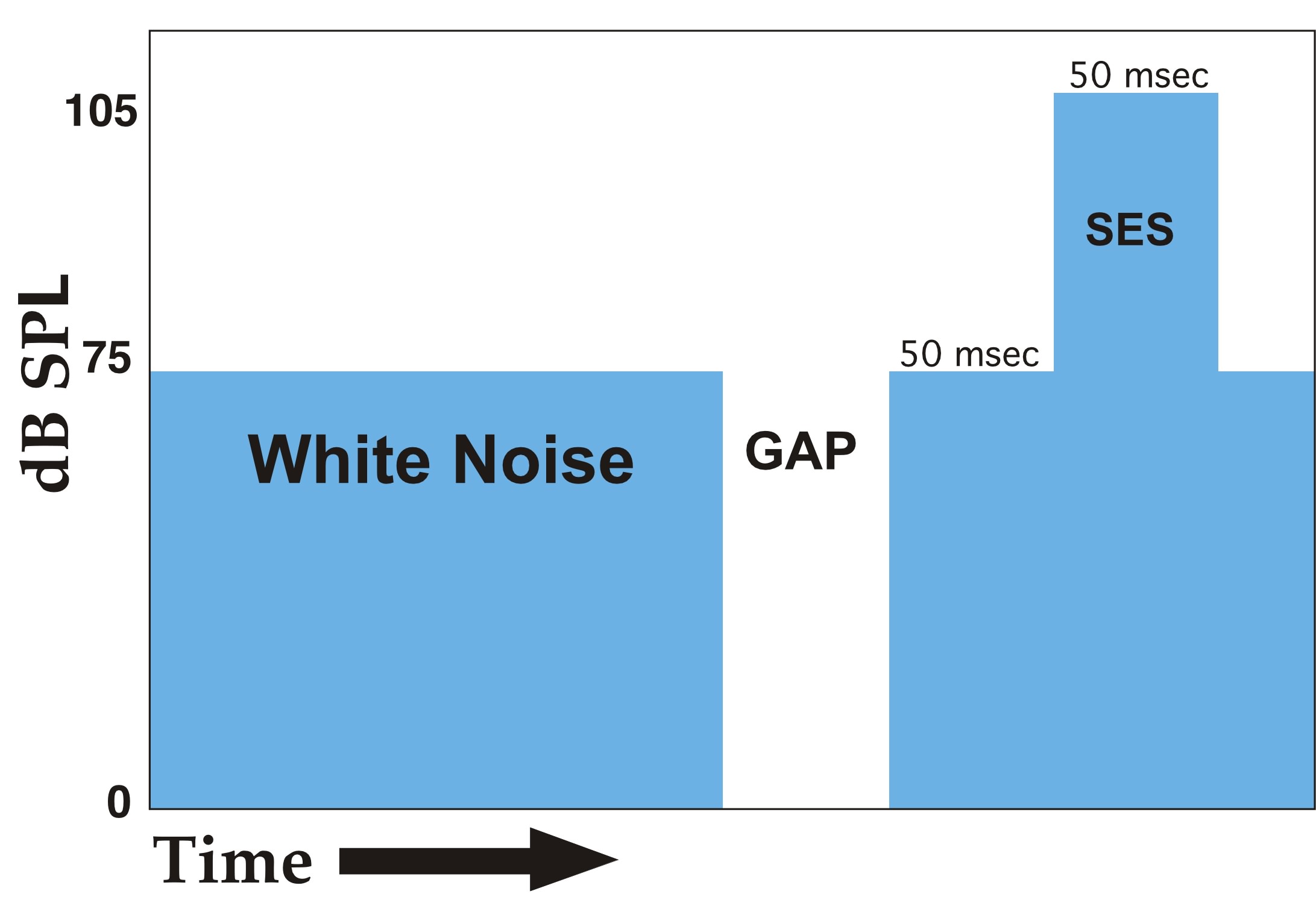 |
Figure 2 - Single trial schema of the gap detection paradigm. The duration of the gap randomly varied between the values of 0 (no gap), 2, 5, 10, 20, 30, 40, and 50 msec across 304 trials. SES = Startle Eliciting Stimulus. |
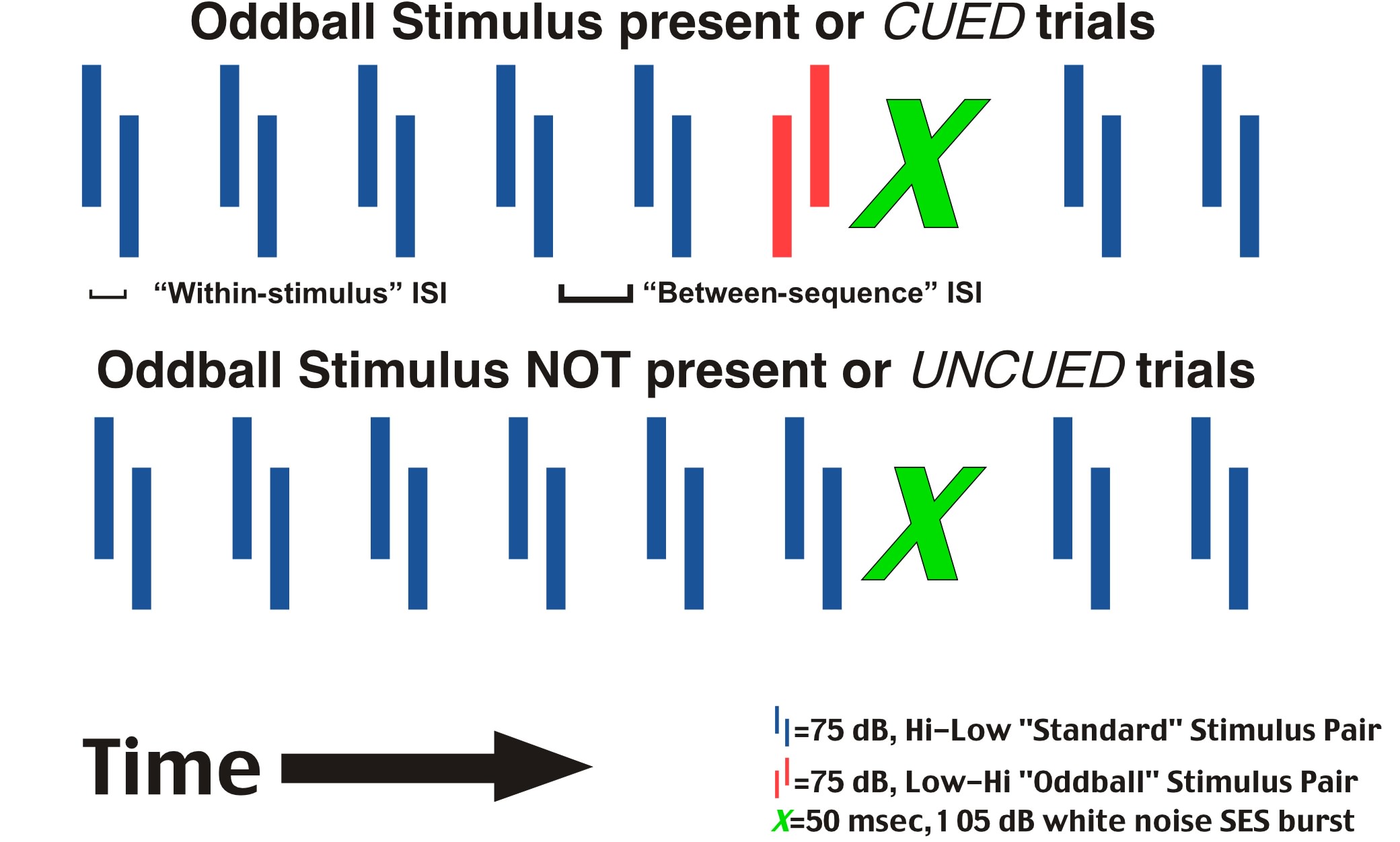
In addition, we sought to adapt the reflex modification paradigm to extend the prior results of Fitch and colleagues (39–42), which showed deficits in processing rapidly presented two-tone stimuli for microgyric male rats (Experiment 2). Towards this end, we adapted an oddball stimulus format to the reflex modification paradigm. Oddball paradigms are consistently utilized in non-behavioral research such as auditory evoked potential and mismatch negativity (MMN) research (see 47,48). In such studies, a significant MMN indicates the detection of an oddball stimulus. In our study, the detection of the oddball is indicated by a significant reduction in startle amplitude when the oddball is present (cued trials), as compared to startle amplitude when the oddball is not present (uncued trials). Recall that in the gap detection paradigm, low-level white noise served as an acoustic “background” with brief intervals of silence immediately preceding the presentation of the startle eliciting stimulus. Now, a repeating two-tone stimulus served as the acoustic “background” and a reversal of the two-tone sequence immediately preceding the startle-eliciting stimulus served as the pre-stimulus cue (Figure 3)
A total of 12 adult male Wistar rats (6 sham, 6 lesioned) were tested in Experiments 1 and 2, and the results are presented below
Post mortem analysis confirmed the presence of bilateral microgyria in the animals exposed to the P1 freezing lesions. These malformations were located in somatosensory-related cortex (SM-I) including regions Par1, Par2, HL, and FL (49). There were no malformations seen in any of the sham subjects.
EXPERIMENT 1
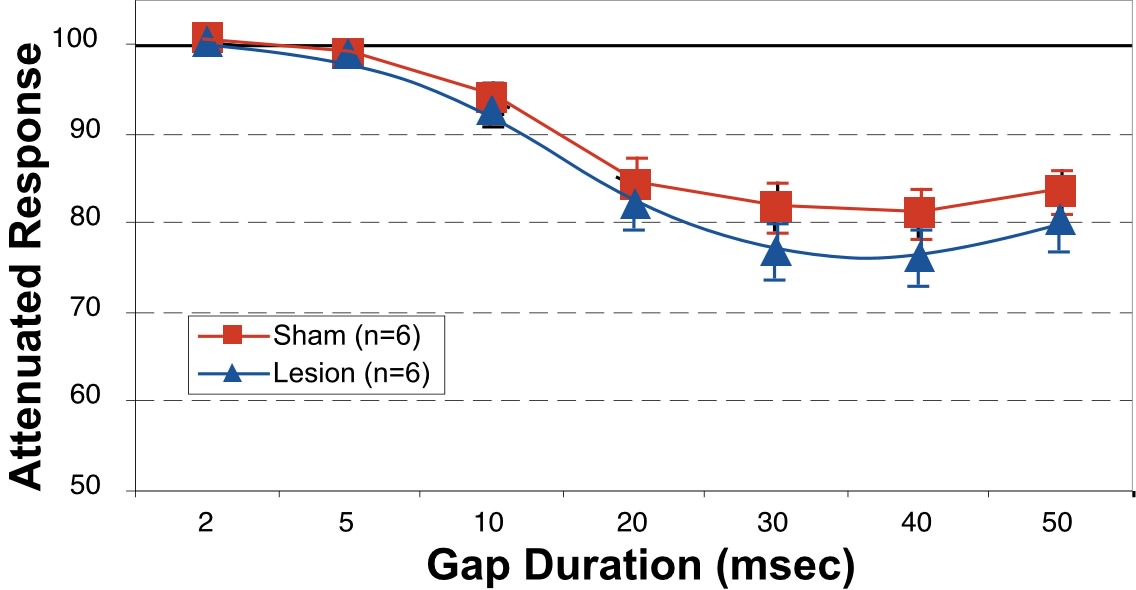
Mean startle amplitudes were computed for each subject at each condition; grand means were subsequently computed for all subjects within a treatment at each condition (Figure 4). An overall Analysis of Variance (ANOVA) was performed on the dependent variable (mean absolute startle response amplitude) within each treatment group (i.e., Lesion or Sham). There was one Within-variable—Gap Duration— with 8 levels (including the 0 msec condition). Absolute reflex response measures for Sham and Lesion rats were analyzed separately to assess whether the rats in the respective groups were exhibiting significant reflex modification at each duration (i.e., differential response to cued vs. uncued trials), which in turn indicates significant detection of the pre-pulse stimulus. In both groups, a significant main effect of Gap Duration was observed (Sham, F7,35=42.55 , p<.0001; Lesion, F7,35=42.49, p<.0001). These analyses were followed by a pair-wise comparison of the average startle response for each subject on cued (i.e., gap durations of 2, 5, 10, 20, 30, 40 and 50 msec) versus uncued (i.e., 0 msec or “no gap”) trials, in order to determine which gap durations produced a significant auditory startle reduction (i.e., were detected). In both Sham and Lesion groups, significant differences between cued and uncued responses were evident at gap durations down to 10 msec, but not for 2 or 5 msec.
Responses were then converted to percentages, specifically representing the cued response as a percentage of baseline (uncued) response for each subject at each gap duration. If no advantage was conferred by a given gap duration (i.e., no detection), the cued response should approximate the uncued one (i.e., 100%). A single ANOVA was performed on these converted measures using Treatment (with 2 levels, Sham and Lesion) as the Between-variable, and Gap Duration as a Within-variable (but with only 7 levels, since all values at the 0 gap condition converted to 100% and this condition was not included). Again, there was a significant effect of Gap Duration (F6,60=81.9, p<.0001). However, no main effect of Treatment was observed (F1,10 < 1, ns), nor was there any interaction of Gap Duration X Treatment (F6,60= < 1, ns). To insure the lack of a Treatment effect at even one Gap Duration, which could be obscured if all other conditions were non-significant, a simple effects analysis of Treatment at each Gap Duration was performed. This confirmed a lack of Treatment effects for any Gap Duration (F1,10 < 1, ns for all durations; Figure 4).
The results from this experiment demonstrate no significant differences between microgyric and sham subjects in detecting a brief gap of silence in an otherwise continuous background white noise. Moreover, the results observed for our sham subjects closely approximate those observed in prior studies with Sprague-Dawley (50) and hooded (51,52) rats. However, we will note that alterations to the stimulus parameters used in the current paradigm might lead to more sensitive assessment of gap detection, and thus ultimately elicit a subtle group difference. For example, Trehub et al. (29) discuss the notion that presentation of silent gaps inserted into a continuous background stimulus (as we have done) may cause neural adaptation, and hence a less robust level of neural activity against which to detect a brief interval of neural decay. In support of this view, she found that silent gaps inserted into brief Gaussian-enveloped tone pips provided a more sensitive index of minimal detectable gap duration for infants than had previously been reported (29). Other stimulus parameters which could be modified include ramping of silent gaps, and use of single-frequency tones as our “background.” These manipulations will be examined in future research.
EXPERIMENT 2
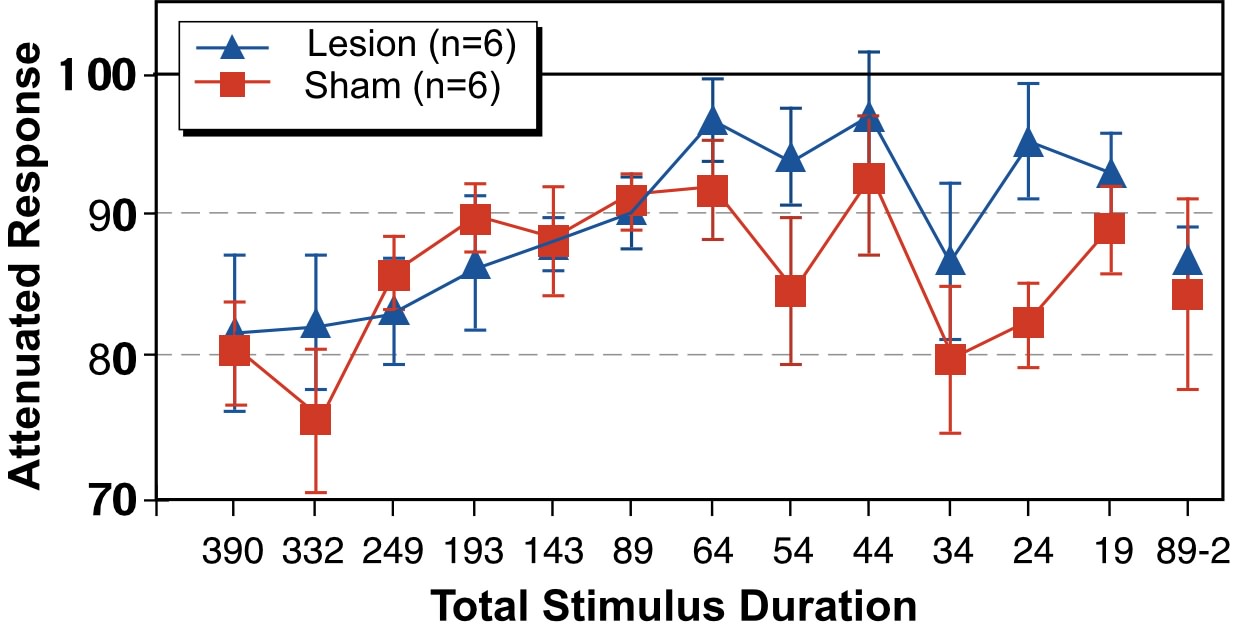
As in Experiment 1, mean startle amplitudes were computed for each subject at each condition. Grand means for percent attenuation of startle within each treatment are shown in Figure 5. We performed ANOVAs on the average startle response for each subject using Cue as a Within-variable with 2 levels (cued/oddball stimulus present, and uncued/ oddball stimulus not present), and Stimulus Duration (i.e., duration of within-tone ISI + tones), a Within-variable with 13 levels. As in Experiment 1, these analyses were performed within the Sham and Lesion groups separately. In the Sham group, a main effect of Cue was observed across all stimulus durations (F1,5=31.5, p<.005). Simple effects revealed a significant difference between cued and uncued responses at virtually every stimulus duration, down to durations of 34 msec (F1,5=11.3, p<.02), 24 msec (F1,5=24, p<.005) and even 19 msec (F1,5=11.7, p<.02). Microgyric subjects also demonstrated a main effect of Cue (F1,5=14.7, p<.02). However, for this group an interaction between Cue X Stimulus Duration was also evident (F12,60=2.1, p<.05). A simple effects analysis revealed a significant difference between cued and uncued responses at stimulus durations down to 89 msec (F1,5=14.9, p<.02), but not at durations of 64, 54, 44, 34, 24 or 19 msec.
Next, cued response was converted to a percentage of uncued response (cued/uncued x 100) for each subject at each stimulus duration, what we have called “attenuated response.” Based on the above results, which show that group performance diverged between 64 and 89 msec stimulus duration, we split our data into two stimulus conditions – one for “short” stimuli (stimulus durations of 64 msec and less) and one for “long” stimuli (stimulus durations of 89 msec and greater). Individual subject responses at each condition within “long” and “short” were averaged to produce a mean Long and Short stimulus duration performance index for each subject. These values were then analyzed using Treatment as a Between-variable with 2 levels (Lesion and Sham), and Stimulus Duration as a within-variable with 2 levels (Long and Short). This analysis revealed a near significant interaction of Treatment X Stimulus Duration, (F1,10=4.89, p=.051). In order to explore this interaction, we further analyzed Treatment at each stimulus duration (Long and Short separately). The between-group analysis at long durations revealed no significant group differences (F1,10<1, ns). However, the analysis of Treatment at short stimulus durations revealed a significant main effect of Treatment (F1,10=6.3, p<.05), with Shams showing better startle reduction (Figure 6). This result was consistent with our finding that Sham, but not Lesion subjects, exhibited significant effects of Cue when absolute responses were analyzed at short stimulus duration conditions.
These results show that microgyric lesioned rats are impaired in their ability to process brief auditory stimuli followed in rapid succession by other acoustic information. That is, microgyric rats demonstrated an impairment in processing auditory stimuli that have both a temporal and spectral demand. Additionally, the current results support prior findings of Fitch and colleagues (39–42), further validating the reflex modification technique as a mechanism to examine complex auditory temporal processing in our animal model.
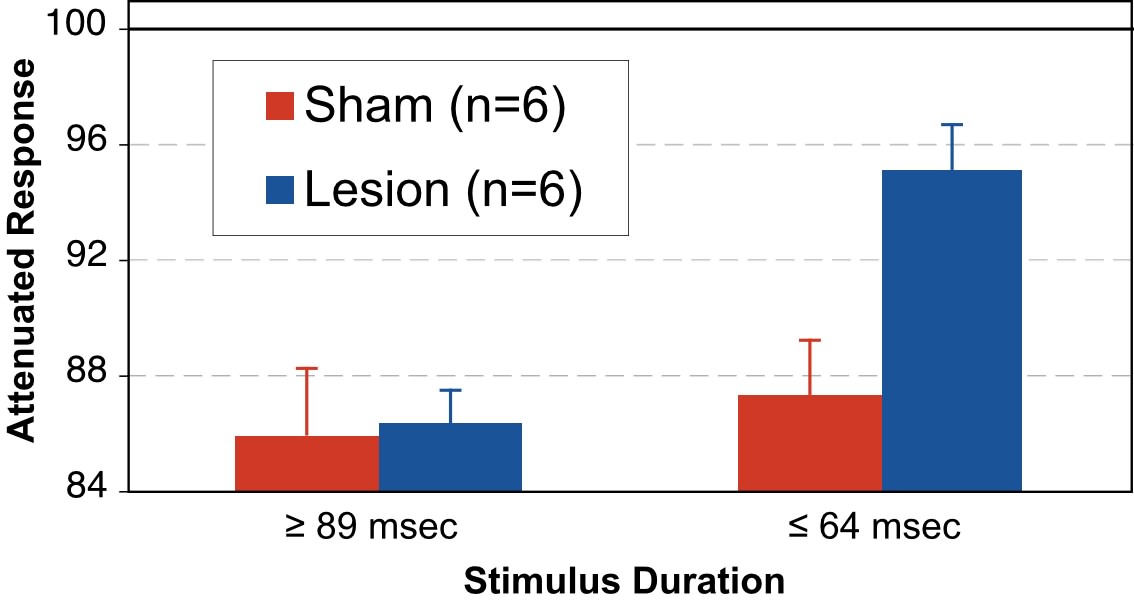 |
Figure 6 - Grand means and SEM of Attenuated Response at the combined “long” (>89 msec) and “short” (<64 msec) stimulus durations. |
The results reported here demonstrate differential performance between adult male rats with induced malformations of cortex versus controls for oddball detection of brief, rapidly-presented stimuli (64 msec or less), using a reflex modification paradigm. In contrast, there was no significant difference between these groups for oddball detection of long-duration stimuli (89 msec or greater) or for detection of single silent gaps in white noise (regardless of duration), in a reflex modification paradigm. These results lead to two important conclusions.
Rapid Auditory Processing Deficits in Rats with Microgyria
We have replicated our previous finding that rats with induced malformations of the cortex exhibit deficits in processing “rapidly changing” auditory stimuli (39–42), and expanded this finding to include the use of a novel paradigm to assess auditory discrimination thresholds. This new result supports the viability of our animal model linking the cortical malformations seen in dyslexic brains with the auditory processing deficits seen in language impaired individuals
While not a direct focus of this study, the question has been raised regarding mechanisms via which bilateral parietal (SM-I) microgyria might affect complex auditory temporal processing in rats. This issue has been addressed in prior work. Specifically, Herman and colleagues (40) reported that adult male rats with bilaterally induced cerebrocortical microgyria in frontal, occipital and parietal cortices had more small and fewer large neurons in the medial geniculate nucleus (MGN) of the thalamus, again irrespective of lesion location. Interestingly, these same subjects were significantly impaired in their ability to discriminate rapidly presented two-tone sequences identical to the those used in the current study. This link between malformations in the cerebral cortex and changes in cell size in the thalamus has also been demonstrated in humans. For example, Galaburda and colleagues (53) found more small and fewer large neurons in the left MGN of the brains of developmental dyslexics (all of whom exhibited minor malformations of the neocortex) when compared to control human brains. Moreover, Livingstone and colleagues (54) demonstrated that dyslexics had difficulty processing fast visual information—a function normally mediated by magnocellular pathways of the visual thalamic nucleus (or lateral geniculate nucleus; LGN). In addition, they found a 27% decrement in the size of magnocellular neurons in the LGN of dyslexics as compared to controls.
This link between cerebrocortical malformations and changes in the distribution of thalamic cells sizes has been hypothesized to be due to connectional changes resulting from early focal injury to the developing neocortex (40). Thus, neonatal freezing lesions may disrupt the formation of not only the afferent and efferent connections associated with the damaged region (e.g. 55, see also 56), but may also result in the maintenance of otherwise transient connections (e.g., 57). There also exists evidence of anomalous cortico-cortical connections formed in association with microgyria, and there is a marked disruption of the reciprocal connections between the affected cortex and the thalamus (58). Taken together, evidence suggests that induced developmental focal anomalies such as microgyria may lead to anatomical changes in the thalamus, which may in turn be related to auditory processing deficits. This hypothesis will continue to be addressed in ongoing research.
Many Factors May Influence Parameters of “Auditory Processing Deficits” Observed in Microgyric Male Rats
Our current data also suggest that stimulus parameters and task demand may both influence the perceptual threshold for group differences in auditory processing between sham and microgyric male rats (or, alternately, the parameters which define impaired processing in microgyric subjects). This inference derives in part from the failure to detect a group difference in thresholds for gap detection, despite significant detection differences observed for the same subjects, in a similar paradigm, but using more complex stimuli (two-tone sequences). Nevertheless, as we have noted, acceptance of the null hypothesis regarding a lack of group differences in gap detection will require further assessment —specifically, replication of the lack of group differences under different conditions (including but not limited to ramping of silent gaps, and use of gaps inserted in single-frequency tones rather than continuous broadband white noise).
Moreover, we leave open the possibility that any interaction between auditory processing deficits, task specificity and stimulus parameters may be age-dependent. This view is supported by evidence that gap detection thresholds significantly predict language development in normal babies (29,30), and by research indicating that gap detection thresholds are elevated in some young children with SLI and reading disability (31,32), even though no differences in gap detection thresholds are seen in adult dyslexics(27,28). Thus, it is possible that gap detection threshold differences might be evident if, for example, we examined very young microgyric and sham subjects. This issue is important in that deficits occurring early in development can have a profound impact on cognitive organization, even if these same deficits are resolved and are no longer observable later in life.
Additionally, the inference that task demand may influence the parameters which define “impaired” performance for microgyric subjects is supported by a marked discrepancy between the threshold where sham/microgyric differences were seen on the two-tone oddball detection task described here (with group differences evident for a total stimulus duration of 64 msec (two 7 msec tones and a 50 msec ISI), versus the threshold for sham/microgyric differences reported previously on a complex two-tone target identification operant task (where group differences were significant at a total stimulus duration of 249 msec (two 12 msec tones and a 225 msec ISI; (39–41). The latter difference was replicated in 5 behavioral studies (40), and was recently replicated using a new variable ISI version of the task (42). Indeed, the subjects in the latter variable ISI operant task (wherein sham/microgyric differences were seen on the discrimination of two-tone stimuli of 249 msec duration), were littermates of the subjects used in the current study. This fact makes the differences in perceptual threshold at which group differences in auditory processing were found—249 msec total stimulus duration on the operant target ID task, versus 64 msec total stimulus duration on an oddball detection task—particularly compelling.
The research literature on auditory processing in children also speaks to this finding by demonstrating that the deficits seen in SLI children for processing two-tone sequences at short ISIs becomes even more marked if additional elements (i.e., 3+) are added to the task (3,7,25). Other research findings have demonstrated that children with SLI do not differ from controls on the discrimination of single 40 msec duration steady-state vowel sounds. However, these same subjects are impaired in discriminating both vowel-vowel diphthong syllables (comprising an initial 40 msec steady-state vowel sound followed in rapid succession by a different vowel sound of 210 msec), as well as consonant-vowel syllables (comprised of a 40 msec differential transitional segment followed by a steady-state vowel of 210 msec; 2,3,25). This finding supports the view that greater cognitive demands from either stimulus properties or increased memory requirements due to an increased number of task elements may interact with, or exacerbate, perceptual processing constraints in language impaired individuals, as well as in our animal model. This fact has critical relevance to the current disputes surrounding the issue of whether processing deficits in language impaired populations represent basic sensory processing deficits (i.e., 22) versus higher-level cognitive ones (i.e., 59,60). Again consistent with this view, behavioral studies on mice with spontaneous neocortical malformations, similar to those seen in dyslexic brains, have shown deficits in both reference and working memory (61,62). Taken together, these findings suggest that a careful psychophysical analysis in which stimulus and cognitive task parameters are manipulated on independent axes, could further elucidate the nature and level of processing deficits, both in language impaired populations and in our animal model, and in turn help to resolve apparent theoretical differences.
In summary, humans with developmental language disabilities exhibit specific deficits in processing successive, rapidly presented, auditory information. It has additionally been demonstrated via postmortem analyses of the brains of dyslexics that they exhibit developmental neuropathologic anomalies, such as developmental microgyria. Previously, we demonstrated that rats with induced microgyric lesions are significantly impaired compared to sham subjects in auditory discrimination of rapidly presented two-tone stimuli. The goal of the current research was to further characterize the extent and nature of this rapid auditory processing deficit in rats with microgyric lesions. Current results confirm that rats with microgyric lesions do have a fundamental dysfunction in the ability to process brief auditory stimuli followed in rapid succession by other acoustic information. Moreover, the observed temporal thresholds in both lesion and sham subjects appear to be related to stimulus complexity and/or cognitive demand. This inference is supported by evidence that microgyric subjects were unimpaired in the detection of brief gaps in broadband white noise, but significantly impaired in processing of short-duration two-tone auditory stimuli. It is also supported by observations of group differences in auditory discrimination at much longer stimulus durations when subjects were tested in a complex operant testing apparatus (39–42) relative to group differences observed in the current study using the same stimuli, but presented in an oddball detection reflex modification paradigm. These results are strikingly similar to the pattern of findings obtained from SLI and dyslexic individuals, and further support the use of an animal model to examine deficits in complex auditory processing, and as a further means to assess a biological basis of developmental language disabilities in humans.
Induction of Focal Necrotic Lesions
Six pregnant female Wistar rats (Charles River Laboratories, Wilmington, MA) were received at the laboratory of GDR. One day after birth (P1), litters were culled to 10 rat pups maximizing for males. Male pups were collected and randomly designated to receive either sham or bilateral freezing lesion surgery balanced within each litter. The focal necrotic lesions were subsequently induced based on a modification of the technique employed by Dvorák and associates (63,64) and explained in detail elsewhere (37,38). In short, pups were anesthetized by hypothermic induction and a midline incision was made over the skull. A cooled (-70°C) 2 mm diameter stainless steel probe was placed on the skull approximately 2 mm lateral of the sagittal suture and 2 mm caudal of bregma for 5 sec, corresponding to the region of the parietal cortex. After placement of the probe, an identical lesion was placed in the opposite hemisphere. The side of the first probe placement was randomly determined. Sham subjects were treated identically with the exception that the steel probe was maintained at room temperature. Following the second lesion, the skin was rapidly sutured, the subjects were marked with ink footpad injections, warmed under a lamp, and returned to the mother.
Litters were weaned on P21 and the subjects group housed (2–3/cage). Twelve subjects, 6 from each treatment group, were then transported to MGC and RHF at the University of Connecticut. Upon receipt, subjects were individually housed in tubs. Throughout the duration of testing, subjects were maintained on a 12-hour light/dark cycle with all testing occurring during the light phase of the cycle. Food and water were available ad libitum, except during the 2 hours of daily testing. Behavioral testing began once the rats had reached adulthood (>70 days of age).
Apparatus
During testing, each subject was placed in an opaque-walled, polypropylene cylindrical cage (25.2 cm dia. ¥ 30.4 cm) atop a Stoelting movement transducer platform, model EAM #31404 (Stoelting Co., Chicago, IL). Four platforms were used and these were located in a quiet testing room (4.5m ¥ 2.3m ¥ 2.7m). The output voltages from the platforms were sent through a band-pass filter with cutoff frequencies of 1000 Hz and 1 Hz, and passed into a Biopac MP100WS Acquisition system (Biopac Systems, Santa Barbara, CA) connected to a Power Macintosh 7200/120, where the signal was rectified on-line. This combined apparatus acts to record the amplitude of the subject’s whole-body acoustic startle reflex. The Biopac system acquired the incoming voltages (representing transduced movement signals) at a frequency of 1000 samples/second throughout a session of testing. The epoch of interest was 150 msec in duration, beginning with the onset of the SES. The extracted peak value during this time frame served as each subject's response amplitude for that trial (our dependent variable).
All auditory stimuli were generated on a Macintosh Quadra 700 computer, and output through two Yamaha YST-M10 powered monitor speakers positioned 75 cm above the platforms. The background white noise was presented at 75 dB SPL. The SES was a 50 msec “burst” of white noise with a 0-msec rise/fall time, presented at 105 dB.
Gap Detection Procedure.
Subjects were individually placed in the cages positioned on the testing platforms. The auditory test paradigm consisted of repeated presentation of the SES with an inter-trial interval (ITI) of 24, 22, 18 or 16 sec, as derived from a paradigm outlined by Leitner and colleagues (50). The ITI was variable to prevent anticipation of the SES. A variable duration silent gap (0–50 msec) was presented 50 msec before the SES, with the gap duration on each trial randomly selected. A given trial (Figure 2), occurring every 20 sec on average, consisted of 75 dB continuous background white noise, the presentation of a silent gap, 50 msec of additional background white noise, followed by presentation of the SES (a 50 msec, 105 dB noise burst). This sequence was immediately repeated for the next trial. Trials that did not contain gaps (i.e., uncued trials) were the same as above but the “gap” was 0 msec in duration.
In this experiment, gap duration represented the independent variable. A complete session contained trials with 0 (no gap), 2, 5, 10, 20,30, 40 and 50 msec gaps. For the purpose of statistical comparison, the 0 msec or “no gap” represented the “uncued” (baseline startle response) condition, while the “cued” conditions included gap durations of 2, 5, 10, 20, 30, 40, and 50 msec. The gaps, like the SES, had a 0 msec rise/fall time. Each of the 8 gap conditions were randomly presented 38 times, for a total of 304 trials during a given test session. All subjects were tested once a day for 5 days.
Oddball Paradigm
The oddball paradigm consisted of the repeated presentation of a “standard” two-tone stimulus. A trial occurred every 16, 18, 22, or 24 sec. (as in Experiment 1), concluded by the presentation of the SES. In half the trials, a standard stimulus was presented 50 msec before the SES. In the remaining trials, an “oddball” stimulus was presented 50 msec before the SES. Trials in which the standard stimulus was presented immediately preceding the SES were considered uncued; trials in which the oddball was presented were considered cued.
The standard stimulus consisted of a high and low tone (2300 Hz and 1100 Hz respectively) of varying duration separated by a variable duration within-stimulus ISI. The two-tone sequences were presented at 75 dB, and the SES was presented at 105 dB, both within the auditory sensitivity range of the rat (65). The sequences were separated by a between-sequence ISI (see Figure 3), which was always 200 msec greater than the within-stimulus ISI to maintain the perceptual contiguity of the tones in the sequence.
The oddball stimulus was comprised of the same tones as the standard stimulus, but presented in reversed order. The within-stimulus and between-sequence ISI were otherwise identical to the standard stimulus. The total stimulus durations utilized across sessions were 390 msec (20 msec tone, 350 msec within-tone ISI, 20 msec tone), 332 msec (16, 300, 16), 249 msec (12, 225, 12), 193 msec (9, 175, 9), 143 msec (9, 125, 9), 89 msec (7, 75, 7), 64 msec (7, 50, 7), 54 msec (7, 40, 7), 44 msec (7, 30, 7), 34 msec (7, 20, 7), 24 msec (7, 10, 7), and 19 msec (7, 5, 7). Subjects were tested on one session per day, with all trials at a given stimulus duration (both standard and oddball). The within-tone ISI and tone duration pairings were presented across test sessions (days) in the order above, followed by one additional test session in which we returned to the 89 msec stimulus condition. Thus, the animals were tested over 13 days (1 day at each of 12 stimulus durations, followed by a retest at the 89-msec condition).
ACKNOWLEDGEMENTS
This research was supported by a grant from the March of Dimes to RHF and by HD20806 and a grant from the New England Branch of the International Dyslexia Association to GDR.