Note to the reader: This is a revised edition of a
paper published in Neuroscience (2001;103:931–939).
The definitive original print version is copyright ©2001 by The International Brain Research Organization.
New figures, text, and links have been
incorporated into the revision. Revised html (http://nervenet.org/netpapers/Rosen/Barrel2001/Barrel.html)
copyright ©2001 by Glenn
D. Rosen
| Click here for a .pdf (250K) copy of the paper |
UNILATERAL INDUCED NEOCORTICAL MALFORMATION AND THE FORMATION OF IPSILATERAL AND CONTRALATERAL BARREL FIELDS
Glenn D. Rosen, Ph.D.*, Heinz Windzio, Albert M. Galaburda, M.D.
Dyslexia Research Laboratory and Charles A. Dana Research Institute, Beth Israel Deaconess Medical Center; Division of Behavioral Neurology , Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston MA 02215; Harvard Medical School, Boston, MA 02115.
*Address Correspondence to:
Glenn D. Rosen
Department of Neurology
Beth Israel Deaconess Medical Center
330 Brookline Ave
Boston, MA 02215
USA
Email: grosen@caregroup.harvard.edu
Freezing lesions to the developing cortical plate of rodents results in a focal malformation resembling human 4-layered microgyria, and this malformation has been shown to result in local and widespread disruptions of neuronal architecture, connectivity, and physiology. Because we had previously demonstrated that microgyria caused disruptions in callosal connections, we hypothesized that freeze lesions to the Postero-Medial Barrel Sub Field (PMBSF) in one hemisphere would affect the organization of this barrel field contralaterally. We placed freeze lesions in the presumptive PMBSF of neonatal rats and, in adulthood, assessed the architecture of the ipsilateral and contralateral barrel fields. Malformations in the PMBSF resulted in substantial decrease in the number of barrels as identified by cytochrome oxidase activity. More importantly, we found an increase in the total area of the contralateral PMBSF, although there was no difference in individual barrel cross-sectional areas, indicating an increase in the area of inter-barrel septae. This increase in the septal area of the contralateral PMBSF is consistent with changes in callosal and/or thalamic connectivity in the contralateral hemisphere. These results are another example of both local and widespread disruption of connectional architecture following induction of focal microgyria.
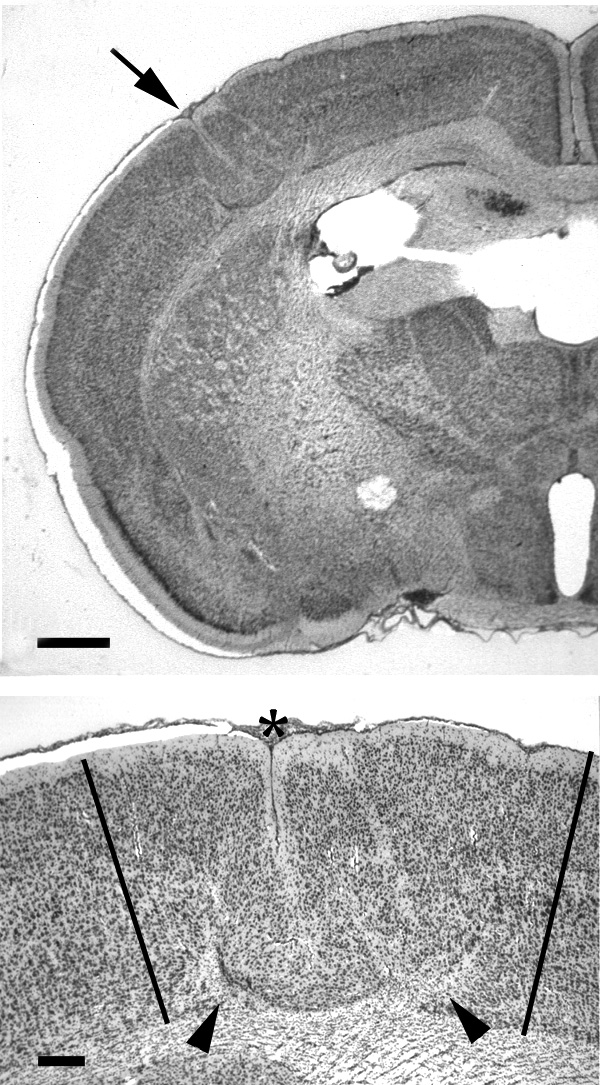
Neocortical malformations have been associated with a wide variety of developmental disorders, including epilepsy and developmental dyslexia (1–3) and these malformations can be induced in otherwise normal rodents through a variety of methods (4–7). For example, freezing lesions to the developing cortical plate of rodents results in a focal malformation resembling human 4-layered microgyria (8–13). This malformation is generally manifested as a focal infolding of the molecular layer of the neocortex forming a microsulcus (layer i), which is surrounded by a cell dense layer contiguous with layers II–III of the neocortex (layer ii). Below this layer is a cell-sparse region (layer iii), and a thin layer iv that is contiguous with the layer VIb (subplate cells) of the neocortex.
In addition to the local distortions of architecture that accompany freeze lesions, widespread effects on other aspects of anatomy, behavior, and neurophysiology have been documented. Male rats with microgyria have difficulties, similar to those seen in individuals with dyslexia, in processing fast auditory stimuli (14–18). Further, this defect in fast auditory processing correlates with a decrement in cell sizes in the medial geniculate nucleus (14,19,20) similar to that reported in dyslexia (21). There are changes in excitatory and inhibitory receptors, both within and outside induced microgyria— N-Methyl-D-Aspartate (NMDA), alpha-amino-3-hydroxy-5-methyl-1,4 isoxazole proprionic acid (AMPA) and kainate receptors are up-regulated, whereas gamma aminobutyric acid (GABA)A and GABAB receptors are down-regulated within the malformation. Outside of the microgyria, AMPA and kainate receptors are up-regulated, whereas GABAA is down-regulated (22). Finally, slices of microgyric cortex have been shown to be epileptogenic—stimulation of the regions directly surrounding the microgyria (the "paramicrogyral zone") result in epileptiform discharges in neocortex as far as 2–4 mm away from it (23–26).
The mechanism whereby this induced focal malformation has such widespread effects on neuronal systems is not yet known, but changes in connectivity associated with developmental injury have been hypothesized to be one potentially important factor (27–28). We have recently demonstrated remarkable changes in connectivity associated with microgyria. Specifically, we have seen an increase in heterotopic callosal projections associated with microgyria, in addition to a near complete absence of thalamocortical fibers within the microgyric region itself. In the paramicrogyral zone, we have seen very dense patches of thalamocortical fibers, which are thought to be responsible for the epileptogenic nature of microgyric cortex (29).
In a recent report, Jacobs et al. (30) reported that a freeze lesion placed into the Postero-Medial Barrel Sub Field (PMBSF) of the rat resulted in substantial disruption of normal architecture of the field. Thus, while PMBSF normally contains 36 barrels (identified by cytochrome oxidase (CO) activity), these researchers found a significant in the number of barrels (an average of 21) when freeze lesions were placed directly into the PMBSF. In addition, they found an increase in CO-activity in the paramicrogyral zone, and this area of CO increase was related to the epileptiform nature of the cortex On the other hand, qualitative examination of the PMBSF of the unlesioned hemisphere of lesioned animals did not reveal any differences from the comparable hemisphere in unlesioned controls.
Because microgyria disrupt the patterns of callosal connectivity, and there are callosal projections that innervate the regions between the barrels (septa) of the PMBSF (31–34), we hypothesized that freeze lesions to the PMBSF in one hemisphere would affect the organization of this barrel field in the contralateral hemisphere, which might be best examined quantitatively. In this study, therefore, we induced microgyria in the PMBSF and qualitatively and quantitatively assessed the changes both in the damaged and undamaged hemispheres.
All efforts were made to minimize both the suffering and number of animals used in this experiment. All experimental procedures conformed to the standards of the Beth Israel Deaconess Medical Center’s Institutional Animal Care and Use Committee.
Pregnant Wistar rats were obtained from Charles River Laboratories (Wilmington, MA) on gestational day 16–18 (E16–18). On the day after birth (P1), animals were randomly assigned to receive a freezing injury to the presumptive whisker barrel fields (PMBSF) of the left hemisphere or a sham surgery. In adulthood, (P60+) animals were perfused transcardially with 0.9% saline followed by 4% paraformaldehyde, and their brains subsequently processed for cytochrome oxidase histochemistry. Barrel fields were drawn using a camera lucida in the lesioned and unlesioned hemispheres. These barrel fields were analyzed qualitatively and quantitatively as outlined below.
Induction of Microgyria
Microgyria were induced based on a modification of a technique by Dvorák and colleagues (8,9), and reported in detail elsewhere (12,13). Pups were anesthetized with hypothermia, and a small incision was made in the anteroposterior plane of the skin over the midline, exposing the skull. A cooled (-70°C) 2 mm diameter stainless steel probe was placed on the skull over the left hemisphere, approximately 2 mm lateral of the sagittal suture and 2 mm caudal of bregma for 5 s. Animals receiving sham surgery were treated identically to those receiving freezing injury except that the probe was maintained at room temperature. After surgery, the skin was quickly sutured, subjects were marked with identifying ink injections to the footpads, warmed under a lamp, and returned to their mother.
Histology
For all perfusions, subjects were anesthetized (pentobarbital, 60 mg/kg i.p.) and were transcardially perfused with 0.9% saline followed by 1.5% paraformaldehyde/2.5% glutaraldehyde. The brains were removed from the skulls and placed into fresh fixative for 24 h. The brains were then placed in a 10% sucrose solution in 0.1 M sodium phosphate buffer for at least 24 hr, and then placed in 30% sucrose buffer until the brains sank. The location on of the malformation was noted before the neocortical hemispheres were individually dissected and flattened between two glass slides and placed in dry ice for 2–4 hours. The flattened hemispheres were then sectioned tangentially on freezing microtome at 80 µm, and the sections were stored in 0.1 M sodium phosphate buffer. Every section (9–15 per brain) was stained for cytochrome oxidase using standard protocols (35).
Analysis
All sections were analyzed under light microscopy. Using a camera lucida attached to Zeiss Universal microscope, the barrel fields were drawn in both the lesioned and unlesioned hemispheres. For most subjects, the barrelfields were visible on more than one consecutive section. In order to align consecutive sections, both barrels and blood vessels were drawn on the initial section. After completing the tracing of these objects, adjacent sections were aligned using both the previously drawn barrels and blood vessels. All sections containing barrels were subsequently traced on the same piece of paper. The number of sections used to draw the barrelfields (2–4) did not differ between lesioned and control groups.
For quantitative analysis of microgyric animals, the area of each barrel of the contralateral PMBSF (PMBSFc ) was measured using NIH Image. For sham animals we measured the PMBSF in both hemispheres. The area of the entire PMBSFc in lesioned, and the PMBSF in both hemispheres of controls was measured using the methods of Riddle and Purves (36). Briefly, this consisted of determining the centroid of each of the barrels along the outside border of the barrelfield, and measuring the area of the polygon drawn connecting these centroids. Chi-square and ANOVA were used to statistically evaluate the data.
As expected, animals receiving a freezing injury to the cortical plate had obvious lesions when examined in adulthood. No malformations were noted in animals receiving sham surgery. An example of a typical malformation is illustrated in Fig 1. The location of the lesions varied somewhat, and animals were categorized as to whether or not their lesions impinged on the ipsilateral PMBSF (PMBSFi). There were 12 animals with lesions in the PMBSF, 3 with lesions outside the PMBSF, and 9 sham subjects.
Qualitative Analysis
The appearance of barrel fields was normal in control animals. An example of a typical section can be seen in Fig 2.
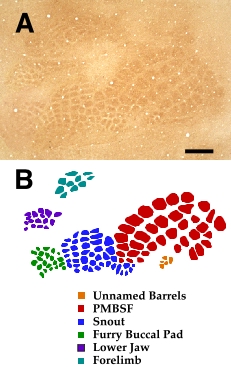 |
| Figure 2 - Cytochrome Oxidase (CO) Activity in Flattened Section. Photomontage of flattened section stained for CO activity (A) and tracing (B) illustrating typical barrel fields in control subjects. Bar = 800 µm |
The PMBSFi is distorted. Not surprisingly, there was substantial disruption of the barrel fields when the lesion was located within them. In some cases, there were no detectable barrels found in the tangential sections. When individual barrels were present, it was difficult to assign them to any one particular barrel field (Fig 3A). When lesions occurred outside the PMBSFi, there was little noticeable disruption of any of the barrel field (Fig 3B).
 |
| Figure 3 - Malformations Within and Outside PMBSFi. A. Drawing of barrel fields from brain with malformation (arrow) located within the PMBSF. Note a near complete absence of all barrels in the PMBSFi. B. Drawing of barrel fields from brain with malformation located outside the PMBSFi. In contrast, these malformations (arrows) do not disturb the normal pattern of barrel formation in the PMBSFi. Orientation arrows: M= medial, A= anterior. |
There are areas of increased CO density in layer ii of the microgyria. In the regions surrounding the microsulcus, there was a uniform increased density of CO, as compared to background, that was visible throughout its depth, and that corresponded to layer ii of the microgyrus (Fig 4). We confirmed this by sectioning an additional 3 microgyric brains in the coronal plane and reacting the tissue for CO as can be seen in Fig 5. Unlike the control subjects, where staining in layer IV is punctuated by regular periods of increased CO density, CO activity in the lesioned subjects was uniformly dense.
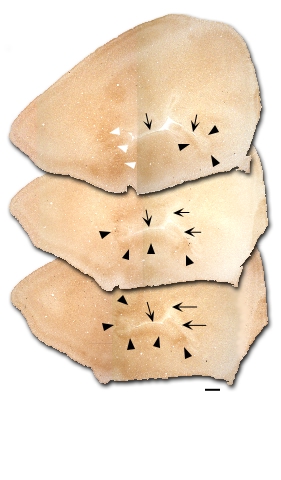 |
| Figure 4 - Cytochrome Oxidase Activity Associated with a Malformation. Three sections from one subject with an induced malformation (arrows) in the PMBSFi. There are patches of dense CO staining (arrowheads), but few, if any barrels visible. Bar = 800 µm. |
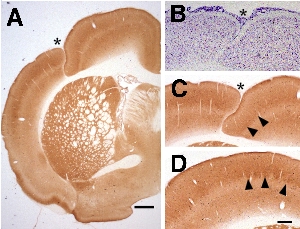 |
| Figure 5 - Cytochrome Oxidase Activity in Layer ii of the Microgyric Malformation. A. Low power photomicrograph of coronally-sectioned CO-stained subject with induced malformation (asterisk). Bar = 800 µm. B. Adjacent Nissl-stained section. C. Higher power photomicrograph of malformation showing a continuous stripe of dense CO activity (arrowheads). D. CO-stained section from control subject showing typical barrel formation (patches of increased CO density). Bar for B-D = 400 µm. |
Quantitative Analysis
All quantitative analysis was undertaken on the PMBSF in the right hemisphere, i.e., the hemisphere contralateral to the malformation (PMBSFc). We chose to examine the PMBSF because it has been previously been shown to be susceptible to developmental intervention (e.g., 37–40).
There are missing barrels in the PMBSFc. The PMBSF has a well-defined structure consisting of 36 individual barrels in five rows (Fig 6). Of the 12 animals with lesions to the PMBSFi, 5 had at least one barrel missing from the PMBSFc. In contrast, there was a single subject with just one barrel missing from the 20 control hemispheres. This difference in distribution was significant (Chi2=6.62, df = 1, P < .05). Of the three animals with malformations located outside of the barrel fields, there was one subject with one missing barrel in the PMBSFc. This distribution did not differ from either of the other groups (Fig 6).
 |
| Figure 6 - Missing Barrels in the PMBSF. A. Drawing of PMBSFc from control subject with each barrel labeled using standard designations. B. Drawing of PMBSFc from subject with missing barrels in the periphery of the field. C. Drawing of PMBSFc from a subject with a missing barrel in the center of the field. D. Drawing of PMBSF from the one control subject with a single missing barrel. |
There is no difference in individual barrel area measurements in the PMBSFc between microgyric and sham animals. We analyzed the area of individual barrels within the whisker barrel field using a one-way ANOVA, with Lesion (PMBSF, Outside PMBSF, Sham) as the independent measure. We found no significant differences among the independent measures for any of the individual barrel areas. We also performed a one-way ANOVA with Lesion dichotomized as either PMBSF or Sham, and again there were no significant differences.Finally, we performed a repeated measures ANOVA, with ‘Lesion’ as the between factor and ‘Barrels’ as within factors. Although there were significant differences between the individual barrels, there were no main effects of Lesion, nor were there any interactions with Lesion. These results are summarized in Fig 7.
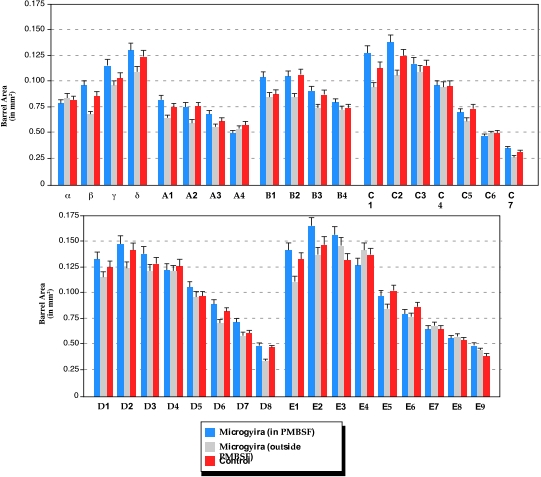 |
| Figure 7 - Mean Size of Individual PMBSFc Barrels. Histograms showing the mean (and SEM) area of each of the barrels for subjects with lesions within the PMBSFi (measures of contralateral barrels, n = 10–12), those with lesions outside the PMBSFi (measures of contralateral barrels. n=2 or 3), and controls (both hemispheres, n=17 or 18). There are no significant differences among the three groups. The number of animals vary because of missing barrels. |
The total PMBSFc area is larger in animals with microgyria in the PMBSFi. Having found no differences in the area of individual barrels in the whisker barrelfield, we then measured the area of the entire barrelfield. Animals with missing barrels were excluded from this analysis, thereby reducing the number of control hemispheres to 17 and the lesioned animals to 7. We analyzed these data using a one-way ANOVA, with Lesion (PMBSF, Sham) as the independent measure. There was a significant effect of Lesion (F2,24 = 4.85, P < 0.05). The area of the PMBSFc in animals with lesions was significantly greater than that of shams (x ± SEM= 4.22 ± 0.29 vs. 3.76 ± 0.07, respectively). These results are summarized in Fig 8.
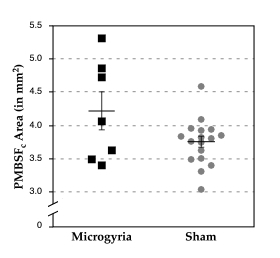 |
| Figure 8 - Total PMBSFc Area in Lesioned and Control Subjects. Scatterplot of total PMBSFc areas for subjects with lesions in the PMBSFi and controls. Bars indicated means ± SEM. The PMBSFc of lesioned subjects are significantly larger than the controls (P < .05). |
Changes to PMBSFi
We found changes in the ipsilateral and contralateral PMBSF. Our findings in the ipsilateral hemisphere are similar to those previously reported (30). As with the previous report, we found that freeze lesions placed into the PMBSFi severely disrupted the formation of barrels (as identified by CO activity) in that field. We also noted the increase in CO-activity in the regions surrounding the malformation, and showed that this increase in CO-activity can be seen in layer ii of the microgyrus.
There were significant differences between our findings in the PMBSFi and those of Jacobs et al. (30). For example, we found a much greater degree of disruption, with most of our cases showing little or no evidence of barrel formation and none having more than 10 barrels. In contrast, Jacobs et al. report the mean number of barrels as 21 ± 2.4. There are a number of possible explanations for this discrepancy. It could be an issue of sensitivity of CO staining, with our method yielding lower contrast between regions of CO-activity and background and therefore making barrel identification difficult. We do not consider this a likely explanation as we show similar patterns of staining of control brains, as well as dense staining of barrels outside the PMBSFi. Another possibility is one of lesion selection. We strictly limited our analysis to those lesions that were placed directly in center of the PMBSFi, and it is not clear whether this strict selection also applied to the previous report. Finally, it could be that our freeze lesions resulted in more severe malformations than those of Jacobs et al., and that these larger malformations had more drastic effects on the local barrel field. Comparison of lesion size from previous publications from this group, as well direct examination of their tissue (41) support this possibility.
Changes to the PMBSFc
In the previous report, Jacobs et al. reported that the appearance of the PMBSFc was normal. This was strictly a qualitative statement. We performed a quantitative analysis and found two differences between the PMBSFc and those of control brains: First, we found missing barrels in the PMBSFc in 5 of 12 subjects with lesions in the PMBSFi, as compared to only 1 of 18 control hemispheres. This was an unexpected finding and one that, despite the statistical significance, is difficult to interpret. Specifically, it is impossible to rule out technical issues playing a role. While the CO method is time-tested, it has been useful only to show positive activity—the lack of CO activity has not classically been used as a definitive diagnostic demonstration of absence of structure and function. When there are large areas of absent CO-activity (as in the PMBSF of the lesioned hemisphere), it is easier to accept the lack of a positive stain as meaning a absence of barrels. When single barrels are unexpectedly missing, however, it becomes harder to unequivocally rule out artifact. It is true that only one barrel was missing in all the control hemispheres, but replication of these findings using other stains (such as succinic dehydrogenase) would give us greater confidence in these results (36). In addition, examination of barreloids in the ventrobasal thalamus may shed light on this issue (42,43).
We also found an increase in the overall area of the PMBSFc in comparison to control PMBSF. This is despite the fact that there was no difference in the size of the individual barrels of the PMBSF between controls and lesioned animals A possible explanation for this difference could be that the changes in the size of the PMBSFc reflect generalized changes to the entire brain as a result of the freezing injury. For example, it could be that the entire brain is larger in microgyric subjects when compared to controls. Although we didn’t measure brain weight in these subjects, we have done so in subsequent studies, and have a found a significant decrease in brain weight in microgyric subjects (unpublished observations), and this decrease is seen at all developmental ages and in both sexes. It is therefore unlikely that the increase in the PMBSFc we report here reflects more generalized changes in brain size. We therefore argue that if there is no difference in individual barrel size, and that overall changes in brain size are in the opposite direction from those seen in the PMBSFc, then the only explanation for the larger total area of the contralateral PMBSF is that the distance between the barrels is greater.
If the inter-barrel distance is larger, why is it so? We hypothesize that it may reflect changes in connectivity. Individual barrels in the PMBSF represent individual whiskers, and the topography of the PMBSF matches the pattern of whiskers on the snout (44,45). The barrels are densely innervated by thalamocortical fibers from the medial division of the ventral basal nucleus (46,47). Callosal connections, as well as those from the posterior nucleus (PO), on the other hand, are absent in the barrels themselves, but are densest in the septa separating the barrels (31–34). In the current experiment, therefore, we postulate that the increase in size of PMBSFc reflects increases in cortico-thalamic projections from PO and/or callosal connectivity.
As described above, there is evidence to suggest that microgyria disrupts connections between the cortex and thalamus as well as between the two hemispheres (29). Thus, we reported the presence of heterologous, as well as a diminution or absence of homologous, connections to the opposite hemisphere following induction of microgyria into various components of the somatosensory cortex. It could be that this decrease in homotopic callosal projections could lead to an increase of PO connections in the vacated space. As yet, however, we have no direct evidence of specific changes in thalamocortical or cortico-cortical connections that are disrupted following lesions of the PMBSF solely. Future experiments will address this issue.
It should be mentioned that the range of values for the area of the PMBSF is about 10–15% less than those reported previously (36). This relatively small discrepancy is most likely due to differences in rodent strain, fixation, and/or histochemistry. It is important to note that all subjects in the current experiment were treated identically, and changes in PMBSF areas were not likely to be related to issues of differential tissue shrinkage.
Conclusions
Placement of a freeze lesion into the PMBSF causes changes to architecture of this barrel field as well as to the contralateral barrel field. We have replicated previous results that demonstrated a significant distortion of the PMBSFi following neonatal freeze lesions placed with it. If anything, in our hands the disruption of the PMBSFi appears to be even greater than previously described. This distortion consists of a near absence of barrels as denoted by CO activity, and dense areas of continuous, as opposed to punctate, CO activity in layer ii of the microgyrus. We have also found an increase in the area of the PMBSFc, when compared with those of controls, despite the fact that there are no differences in size of individual barrels. We hypothesize that this difference in PMBSFc reflects changes occurring in the inter-barrel septae, a region known to contain projections from ipsilateral PO and contralateral homotopic cortex. Taken together, these results lend further support to the notion that focal induced malformations can result in widespread distortions of neuronal architecture.
ACKNOWLEDGEMENTS
This work was supported, in part, by grant HD 20806 from the Public Health Service of the USA. The authors wish to acknowledge the technical support of Alison Frank.