Note to the reader: This is a revised edition of a paper published in Developmental Brain Research (1994;82:127–35). The definitive original print version is available from Elsevier Press.
New figures, text, and links have been incorporated into the revision. Revised HTML (http://www.nervenet.org/netpapers/Rosen/Radial94/Radial.html) copyright ©1999 by Glenn D. Rosen
RADIAL GLIA IN THE NEOCORTEX OF ADULT RATS: EFFECTS OF NEONATAL BRAIN INJURY
Glenn D. Rosen, Gordon F. Sherman, & Albert M. Galaburda
Dyslexia Research Laboratory, Beth Israel Deaconess Medical Center; Division of Behavioral Neurology , Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston MA 02215; Harvard Medical School, Boston, MA 02115.
All correspondence should be addressed to:
Glenn Rosen, Ph.D.
Department of Neurology
Beth Israel Deaconess Medical Center
330 Brookline Ave
Boston, MA 02215
Email:grosen@caregroup.harvard.edu
Microgyria can be induced in otherwise normal rat neocortex by a freezing injury to the cortical plate before the completion of neuronal migration. We had previously reported radial glial like-immunoreactive fibers in the area of the microgyria in 32 day-old rats. Here we demonstrate that these glial fibers, which are immunoreactive to Rat-401, vimentin, and glial fibrillary acidic protein (GFAP) antibodies, are seen in adult rats. The appearance of these fibers is hypothesized to result from the release of a trophic factor during the recovery from neonatal injury which acts to either (1) halt the transformation of radial glia to astrocytes and/or dedifferentiate already committed astrocytes, (2) create a hybrid cell, or (3) induce increased proliferation of glia.
Freezing injury to the cortical plate of the newborn rat results in a cerebrocortical malformation that resembles human 4-layered microgyria (1-4). This microgyria is induced only when injury occurs before neuronal migration to the neocortex is complete, e.g., postnatal day (P) 4 (1). In previous experiments it was demonstrated that the formation of the microgyria was the result of brain repair mechanisms occurring during the course of development (5, 6).
We had previously noted that glial fibers immunopositive for glial fibrillary acidic protein (GFAP), vimentin, and Rat-401 and with the morphological appearance of radial glial cells were present in the area of the malformation in P32 rats (6). There was no evidence of radial glial-like immunoreactivity in any other brain region at this age. Normally, radial glial cells transform to astrocytes by P21 (7-10), therefore suggesting that the appearance of these fibers in the P32 rat was abnormal. It could be, however, that the freezing lesion simply acts to delay the transformation of radial glial cells to astrocytes but that eventually all radial glial cells would transform. The present experiment was designed to test this hypothesis and to describe the appearance of the glial fibers in adulthood.
Protocol
A pregnant Wistar rat (Charles River Laboratories, Wilmington, MA) was obtained on days 16-18 of gestation. Focal necrotic lesions were induced on the day of birth (P0) in the cerebral cortex of the pups from this mother using a modification of the technique employed by Dvorák and colleagues(1, 2) and described in detail elsewhere (3, 6). Briefly, pups were anesthetized (hypothermia) and a small incision was made over the left cerebral hemisphere. A stainless steel probe (2 mm diameter at the tip) contained within a 50 ml centrifuge tube filled with methyl butane was cooled to -70°C with dry ice and applied to the skull for 5 seconds. The skin was then quickly sutured, the animal warmed under a lamp, and returned to the mother. Nine animals were sacrificed on either P47, P63, or P99 (n=3 at each age) under deep anesthesia (Pentobarbital, 37.5 mg/kg i.p.) by intracardiac perfusion of 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer. Following perfusion, the brains were removed from the skulls, postfixed for 24 hr in the fixative, placed in a 10% sucrose solution in 0.1 M sodium phosphate buffer for at least 24 hr, and then placed in 30% sucrose buffer until the brains sank. The brains were frozen on dry ice, serially sectioned in the coronal plane at 30 µm, and stored in 0.1 M sodium phosphate buffer. One series of every tenth section was stained with Thionin for Nissl substance. Adjacent series were stained for GFAP, Rat-401, or Vimentin.
Immunohistochemistry
For all antibodies, a control series was run with the omission of the primary antibody and negative staining was seen. All immunohistochemistry was carried out in an identical manner to that previously reported (6).
Glial Fibrillary Acidic Protein
Free-floating sections were rinsed twice in phosphate-buffered saline (PBS; pH 7.4) for five min each and transferred to a buffered 0.6% hydrogen peroxide solution to block staining of endogenous peroxidases. Sections were then placed in vehicle only for 20 min at room temperature. Sections were placed into a 1/25 solution of primary antibody (Incstar, Stillwater, MN) overnight at 4°C. The vehicle (diluent) for all antibody incubations was 5% goat serum in PBS. The next day, sections were transferred into a biotinylated goat anti-rabbit immunoglobulin solution (Vector Laboratories, Burlingame, CA) diluted 1/60 for two hr at room temperature. After two washes in PBS, the sections were placed into ABC complex (Vector Laboratories) for two hr at room temperature. The tissue was rinsed twice in PBS and twice in 50 mM Tris buffer (pH 7.6) and developed, dehydrated, counterstained, and coverslipped.
Rat-401
This monoclonal antibody is directed against radial glial fibers and was provided by S.J. Hockfield, Yale University. Free-floating sections were rinsed twice in phosphate-buffered saline (PBS; pH 7.4) for five min each and transferred to a buffered 0.6% hydrogen peroxide solution to block staining of endogenous peroxidases. The sections were rinsed twice in PBS and incubated overnight at 4°C in a 1/4 dilution of the primary antibody. The diluent for all antibody incubations was 3% rabbit serum in PBS.
Sections were then placed into a solution containing the linking antibody (rabbit anti-mouse immunoglobulin [Dakopatts Z259, Santa Barbara, CA] diluted 1/20) at room temperature for two hr. The sections were rinsed twice with PBS and placed in a 1/250 dilution of mouse peroxidase anti-peroxidase (Dakopatts B650) at room temperature for two hr. The tissue was rinsed twice in PBS and then twice in 50 mM Tris buffer (pH 7.6) and developed using 0.05% diaminobenzidine and 0.005% hydrogen peroxide diluted in Tris. After rinsing with Tris, sections were mounted, dehydrated, counterstained and coverslipped.
Vimentin
This monoclonal antibody (Boehringer Mannheim, Indianapolis, IN) stains radial glial fibers and is somewhat less specific than Rat-401, staining astroglial filaments late during development. The protocol is the same as that for Rat-401 with the exception of the dilution (1/500) of the primary antibody.
Analysis
Nissl-stained sections were examined and the presence of microgyria or other neocortical malformations noted. Adjacent immunohistochemically-stained sections were then examined for the presence of long fibers with the morphology of radial glia both in the area of the lesion, in the contralateral (undamaged) hemisphere, and in undamaged areas of the ipsilateral hemisphere. The numbers of fibers were counted, when present, on each section.
In all nine brains, freezing lesions resulted in the formation of microgyria and other neocortical malformations, such as focal dysplasias and molecular layer ectopias (Figure 1A). In each case, glial fibers immunopositive for GFAP, vimentin, or Rat-401 with radial glial morphology were found only in the area of the malformation. There were no similar fibers found in either the contralateral hemisphere, or undamaged regions of the ipsilateral hemisphere. The abnormal fibers were usually associated with microsulci (Figure 1B, C, and D) or, in the case of GFAP-positive fibers, also attached and perpendicular to the pial surface (Figure 1B). In many cases, it was possible to trace individual immuno-positive fibers in the region of microgyria for more than 250 µm in the plane of section (Figure 2). Most fibers associated with microgyria resembled defasciculated radial glial fibers seen normally during the first week of life in the rat (11), although there were instances of fasciculated fibers being present (see Fig 3).
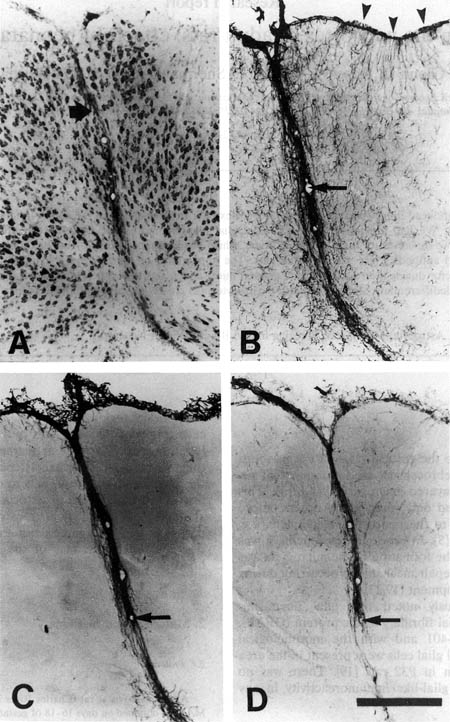 |
Figure 1. A. Nissl-stained section of area of neocortical malformation in a P99 rat induced by placement of freezing probe on the skull of the rat at P1. Note microsulcus (large arrow). B. Section adjacent to that in panel A immunohistochemically stained for GFAP illustrating dense relatively dense staining for astrocytes as well as immuno-positive long glial fibers hanging perpendicular to the pial surface (arrowheads), and at the base of the microsulcus. Arrow in blood vessel is for orientation with Figure 2A. C. Section adjacent to that in panel B immunohistochemically stained for vimentin. Arrow in blood vessel is for orientation with Figure 2B. D. Section adjacent to that in panel C immunohistochemically stained for Rat-401. Arrow in blood vessel is for orientation with Figure 2B. Bar for all panels = 250 µm.
[click here or on figure for an enlarged version (83K)] |
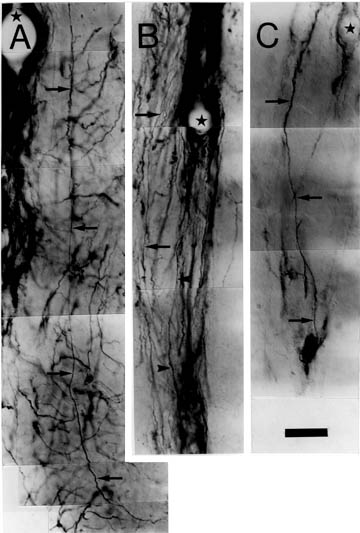 |
Figure 2. A. Photomontage of GFAP-immunopositive fiber with radial glial morphology (arrows). Star is for orientation with arrow in Figure 1B. B. Photomontage of vimentin-immunopositive fibers with radial glial morphology (arrows and arrow heads). Star is for orientation with arrow in Figure 1C. C. Photomontage of Rat-401-immunopositive fiber with radial glial morphology (arrows). Star is for orientation with arrow in Figure 1D. Bar for all panels = 25 µm.
|
|
|
|
|
 |
 |
 |
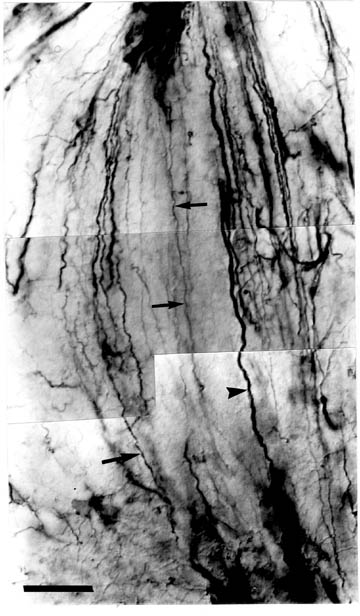
| Figure 3. Vimentin-positive long glial fibers from a rat sacrificed at P63. These fibers have the appearance of defasciculated (arrows) and fasciculated (arrowheads) radial glial fibers. Bar = 20 µm. [click here or on figure to see and enlarged version (61K)] |
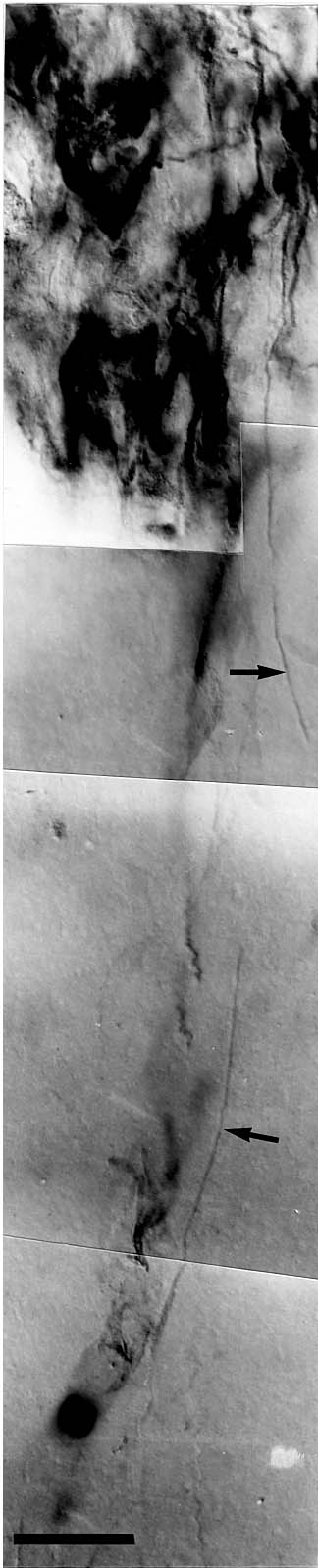
Figure 4. GFAP-positive long glial fibers from a rat sacrificed at P47. Note the large number of fibers as well as the difference in their relative thickness (arrows vs. arrowheads). Bar = 20 µm. [click here or on figure to see and enlarged version (52K)] |
Figure 5. Rat-401-positive long glial fibers from a rat (different from that depicted in Fig 3) sacrificed at P63. Note that the relative paucity of fibers present in comparison to Figs 3 and 4 (see also Fig 2). Bar = 20 µm. [click here or on figure to see and enlarged version (57K)] |
There were large numbers of vimentin- and GFAP-positive fibers with a radial glial-like morphology in these microgyric animals (Figs 4 and 5). In each brain, Vimentin-positive fibers were seen in 3-5 serial sections with each section containing 10-50 fasciculated and fibers (Fig 3), while GFAP-positive fibers were seen in 3-7 serial sections with anywhere from 20-100 or more fibers in each section (Fig 4). In contrast, there were relatively few Rat-401-positive fibers. Specifically, Rat-401-positive fibers were generally seen in 1–3 serial sections, and in each section no more than 2–10 fibers were seen (Fig 5). In addition, these fibers appeared less "kinked" than GFAP- and vimentin-positive fibers. Finally, there was no noticeable change in the numbers of fibers present between the youngest (P47) and oldest (P99) brains examined.
DISCUSSION
In the current experiment, we demonstrate fibers immunopositive for GFAP, vimentin, and Rat-401 with the morphologic appearance of radial glia in adult rats with neonatally induced microgyria. These fibers are relatively few in number and located only in and around the area of the neocortical malformation. These findings raise a number of questions concerning both cortical ontogenesis in general and the response to injury of the immature neocortex in specific which we address below.
Delay in transformation?
Radial glia cells play an essential role in the migration to the neocortex of newly born neurons. Upon leaving the ventricular zone, postmitotic neocortical neurons migrate to the cortical plate using radial glial cells as guides (12). During this stage, radial glia cells are immunoreactive for vimentin and Rat-401, but not for GFAP. In the rat, neocortical migration is completed by P4, at which time radial glial cells then accelerate their transformation to type 1 astrocytes. Most radial glial cells disappear by P10 and there is no trace of them in the neocortex by P21. With the disappearance of radial glial fibers by the third week of life, vimentin- and GFAP-positive staining is seen with mature astrocyte morphology, and there are astrocytes with neither the morphology nor the specific immunoreactivity of radial glial fibers in the cortex (7, 9).
In a previous study, we reported the presence of radial glial fibers in P32 rats that had received a freezing injury to the neocortex at P0 leading to the formation of microgyria. Although this was at a time when fibers immunoreactive for Rat-401 are no longer present normally in the mammalian cortex, there remained the possibility that the freezing injury only delayed the transformation to type-1 astrocytes. With fibers still immunoreactive for Rat-401 on P99, and with no change in the numbers of these fibers at the various postnatal ages, this is not a likely explanation.
Persistence of glial fibers in adulthood
The finding of radial glia in adulthood is not unique to the present observation. Non-mammalian species, including, for example, birds, lizards, and amphibians have radial glia in the CNS well into adulthood (13-16). In addition, the velum medulare of adult monkeys also contains radial glial cells in adulthood as determined by their Golgi-stained morphology (17). The current experiment, however, is the first demonstration of glia with radial glia-like immunoreactivity and morphology in the neocortex of the adult rat. These results are particularly striking in view of the fact that Rat-401 immunoreactivity is not normally seen in the adult CNS (18), although it can be found in Schwann cells of the PNS (19).
The presence of radial glial-like fibers in the adult brain raises two questions: How are the radial glia originally formed and why do they persist into adulthood? As mentioned above, radial glial cells transform to astrocytes starting around the first week after birth, with disappearance of radial glial fibers by the third week (7-10)—a finding which we have confirmed (6). It is not known whether the immunoreactive fibers described in the present study represent normal radial glia or whether they are a hybrid between astrocytes and radial glia produced by this freezing injury.
The possibility also exists that the normal one-way transformation of radial glia to mature astrocytes is disrupted, resulting in a cell that has the antigenicity of both radial glia and astrocytes. In order to determine what might be preventing transformation from radial glia to type 1 astrocytes, it would be useful to understand the mechanisms of transformation in normal development. Currently, however, little is known of the cellular or molecular events subserving this transformation, although there is evidence that interactions with neurons regulate the proliferation and cell shape of glia (e.g. Ref 8). Thus, it could be that there are factors produced by the migrating neurons’ interaction with radial glia that help induce transformation of the latter—either by preventing transformation during the process of migration or by inducing it after migration is complete. We would therefore hypothesize that the freezing injury acts to block release of this factor locally— either directly or indirectly—resulting in the lack of transformation of the radial glia.
One possible scenario whereby this might occur is supported by the finding that layer II of induced microgyria (the layer of cells directly below the molecular layer and above the lamina dissecans) is composed of late-generated neurons (2, 5), specifically those generated on E17 or later (unpublished observations). It could be that these late-generated neurons migrate along radial glial fibers but never actually complete their migration because of the damage to the cortex and as a result, maintain their attachment to the radial glial fibers. If it is the loss of neuronal attachment to glial fibers that triggers the transformation from radial glial to type 1 astrocyte (10), then this would be a possible mechanism for the maintenance of radial glial morphology and antigenicity in this system. We have yet to examine microgyria under EM in order to determine if neurons and radial glia are still apposed to one another in adulthood.
Relatedly, there is evidence to suggest that factors present in embryonic tissue can de-differentiate previously committed astrocytes. Thus, Hunter et al. have shown mouse type 1 astrocytes (both in vivo and in vitro) will de-differentiate to a radial glial morphology and antigenicity in the presence of embryonic cortical neurons (20). Sotelo et al. (21) recently reported that the grafting of embryonic cerebellar tissue into an adult host induced the host’s Bergmann fibers to re-express Rat-401 transiently. Moreover, the Purkinje cells from the donor migrated along these Bergmann fibers suggesting that these fibers not only express the developmentally early antigens in adulthood, but perform functions similar to those seen during ontogenesis. These results support the notion that the presence of embryonic neurons can trigger molecular changes in adult host glia in order that the latter would support migration. In the current experiment, then, it could be that because of the influx of new crops of migrating neurons (see above), type 1 astrocytes might de-differentiate to radial glia and take the place of radial glia that have been destroyed by the freezing injury. It should be pointed out, however, that there are important differences between the current experiments and those cited above. The re-expression of Rat-401 in the Bergmann fibers(21) and the de-differentiation of cortical astrocytes (20) is transient. In former case, for example, the expression of Rat-401 occurs only until the Purkinje cells migrate to their final positions (21). In the current experiment, however, the glial cells maintain their ontogenetic antigenicity and morphology well into adulthood.
Finally, there could be an increase in proliferation of glioblasts giving rise to excess radial glial cells as a result of the damage, perhaps through the release of trophic factors (22-24), Or, conversely, the damage to neurons with subsequent decrease in neuronal numbers could lead to inadequate amount of signaling from neurons for glia to transform. In these two cases, there would occur a change in the glia/neuron ratio, which might lead to diminished transformation of radial glial cells to astrocytes.
The question remains as to what role, if any, the abnormal glial fibers play in the organization of the adult neocortex. The most likely answer is that they are simply remnants of the repair process affecting of the injured cortex, and therefore serve no function in adulthood. Thus, they may have served a purpose during that stage of brain development—as guides for late migrating neurons into the damaged area (see above, Ref 6) as aids in the repair of the glial limitans, or as guides for callosal or other fibers (25)—but are simply vestigial.
Conclusion
The induction of microgyria by freezing injury to the newborn rat cortex results in the maintenance, in adulthood, of fibers that have the morphology and antigenicity of radial glia and that are located only in the damaged area. This is the first documented case of radial glia-like fibers in the neocortex of a mammal. The mechanism for the maintenance of this immature form of glia is, at present, unknown, but we speculate that the freezing lesion acts, directly or indirectly, to either (1) halt the transformation of radial glia to astrocytes and/or dedifferentiate already committed astrocytes to their earlier phenotype, (2) create a hybrid cell with features of both astrocytes and radial glia, or (3) destroy young neurons and induce increased proliferation of all types of glial cells leading to incomplete maturation of radial glia
ACKNOWLEDGMENTS
We would like to thank S.J. Hockfield for generously supplying the Rat-401 antibody, and J.M. Richman and A. Zalkalns for technical assistance. In addition, we thank the anonymous reviewer for his/her thoughtful comments on an earlier version of the manuscript. This work was supported, in part, by PHS Grant HD 20806.