Note to the reader: This is a revised edition of a paper published in Neuroscience (1995;169:107–114). The definitive original print version is available from Elsevier Press.
New figures, text, and links have been incorporated into the revision. Revised HTML (http://www.nervenet.org/netpapers/Rosen/Protect95/Protect.html) copyright ©1999 by Glenn D. Rosen
THE NEUROPROTECTIVE EFFECTS OF MK-801 ON THE INDUCTION OF MICROGYRIA BY FREEZING INJURY TO THE NEWBORN RAT NEOCORTEX
Glenn D. Rosen, Eric A. Sigel, Gordon F. Sherman, & Albert M. Galaburda
Dyslexia Research Laboratory, Beth Israel Deaconess Medical Center; Division of Behavioral Neurology , Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston MA 02215; Harvard Medical School, Boston, MA 02115.
Address Correspondence to:
Glenn Rosen
Department of Neurology
Beth Israel Deaconess Medical Center
330 Brookline Ave.
Boston, MA 02215
Email:grosen@caregroup.harvard.edu
Four-layered microgyria is associated with many developmental disorders, including mental retardation, epilepsy, and developmental dyslexia. Freezing lesions to the newborn rodent neocortex result in the formation of 4-layered microgyria. Previous research had suggested this type of injury acts as an hypoxic/ischemic event to the developing cortical plate. The current study examines the effectiveness of the non-competitive N-Methyl-D-aspartate receptor antagonist dizocilpine (MK-801) in protecting against freezing injury to the newborn rat cortical plate.
Three groups of rats received freezing injury to the cortical plate on the first day of life (P1). Two groups were treated with MK-801 (1 or 2 mg/kg) 0.5 hours before the lesion and 6 and 14 hours after, while one group received saline injections. A fourth group received MK-801 injections, but did not have a freezing lesion. The volume of neocortical abnormality was determined for all three groups in rats sacrificed after P7. Treatment with the higher dose of MK-801 (3 X 2 mg/kg) dramatically reduced the effects of freezing injury. but also resulted in over 50% mortality in both lesioned and unlesioned groups. Animals in the lesioned group, however, had a decreased volume of abnormal cortex, and there were fewer animals with microsulci than in the untreated group. This is the first demonstration of a significant anatomical neuroprotective effect in newborns (P1) leading to a reduction of cortical malformation.
Neuronal death following experimental ischemic/hypoxic events in the brain can be ameliorated by pre- and post-injury treatment with a variety of neuroprotective agents (c.f., Ref 1). Although pharmacologically distinct, these agents have in common their action to block the cascade of neurotoxic steps that follow hypoxic/ischemic injury (2). For example, dizocilpine (MK-801) has been shown to be an effective neuroprotectant through its actions as a non-competitive N-Methyl-D-aspartate (NMDA) receptor antagonist (c.f., Refs 3-5), although there is some question as to the mechanism of action (6,7), as well as about the effectiveness of this agent (8,9). Nonetheless, MK-801 has been shown to ameliorate the neuronal damage associated with transient forebrain ischemia in rats as young as 7 days-of-age (P7; Refs 10-12) and the behavioral effects of anoxia at P1 (13).
Freezing injury to the cortical plate of the newborn rat results in the formation of a focal region of cerebrocortical microdysgenesis resembling human 4-layered microgyria (14-19), a type of malformation observed in association with several human developmental brain disorders (20-27). That experimental microgyria can be induced via injury supports the notion that damage to the developing brain is a key pathogenetic factor in the spontaneous appearance of these types of malformations (26, 28-30). We have previously reported a disruption of glutamatergic fibers in the area of microgyria (neuronal types known to be susceptible to hypoxic events; Ref 31)—in contrast to the relative preservation of VIP-positive immunoreactivity (neurons which are less likely to be disrupted by hypoxia, Refs 32,33)—which led us to the hypothesis that the neuropathologic lesion resulting from early freezing injury was most likely caused by vascular, ischemic damage (18). Thus, treatment with neuroprotective agents might significantly affect the formation of the neocortical malformations which follow neonatal freezing injury. In the current experiment, we examine the effects of MK-801 treatment on the formation of microgyria following a freezing lesion to the newborn rat cortical plate.
Protocol
Pregnant Wistar rats (Charles River Laboratories, Wilmington, MA) were obtained on days 16-18 of gestation. On the day of birth, the pups from these litters were assigned to one of four experimental treatment groups (see Table 1). All rats were pretreated with either MK-801 or saline, anesthetized by hypothermia and half an hour later received either a freezing lesion or sham surgery. Animals were returned to their mothers and post-lesion injections of either MK-801 or saline were given 6 and 14 hours later. Animals were deeply anesthetized with Pentobarbital (37.5 mg/kg i.p.) and were sacrificed at various ages (P7-P147) by transcardial perfusion with saline followed by 4% phosphate-buffered paraformaldehyde (pH 7.4). The brains were removed from the skulls, post-fixed for 24 hours, serially sectioned coronally at 30 µm, mounted onto slides, stained with thionin, and coverslipped with Permount.
A total of 36 surviving animals were used in this experiment.
Experimental Group |
Pre-lesion |
Freezing Injury |
Post-lesion |
| MK1/Les (1 mg/kg) | MK-801 | + | MK-801 |
| MK2/Les (2 mg/kg) | MK-801 | + | MK-801 |
| MK/NoLes (1 or 2 mg/kg) | MK-801 | - | MK-801 |
| NoMK/Les | Saline | + | Saline |
Pre-lesion injection was administered 0.5 h before freezing injury.
Post-lesion injections were given 6 and 14 h following freezing injury.
Neuroprotective and Vehicle Injections
Treatment group MK1/Les received 1 mg/kg MK-801 (provided by Merck & Co, Rahway, NJ; 1 mg/ml) i.p. at each of the 3 injection timepoints and a lesion (see below). Treatment group MK2/Les received a 2 mg/kg MK-801 (2 mg/ml) i.p. injection at each of the 3 timepoints and a lesion, while group MK/NoLes received either 1 (N=1) or 2 mg/Kg (N=12) MK-801 but no lesion. Treatment group NoMK/Les received 0.9% saline (vehicle) injections at each of the 3 timepoints and a lesion.
Freezing Lesions and Sham Surgery
Focal necrotic lesions were induced in animals from treatment groups MK1/Les, MK2/Les, and NoMK/Les based on a modification of the technique employed by Dvorák and colleagues (14,15), and reported in detail elsewhere (17,18). Briefly, pups are anesthetized via induction of hypothermia by immersion in chipped ice for two min. A small incision is made in the antero-posterior plane of the skin over the left cerebral hemisphere, exposing the skull. For those animals receiving freezing lesions, a cooled (~ -70°C) 2 mm diameter stainless steel probe is placed on the skull approximately midway between bregma and lambda, for 5 seconds. Sham subjects (MK/NoLes) are prepared as above, except that the probe is maintained at room temperature. After placement of the probe the skin is quickly sutured, subjects are uniquely marked with ink injections to the footpads, warmed under a lamp until normothermic body temperature is achieved (usually 15 min), and returned to the mother.
Analysis
Qualitative
A distinguishing feature of induced focal microgyria is the formation of a microsulcus. For each brain, it was determined whether a microsulcus had been formed, thereby giving an index to the severity of the cortical reorganization following the freezing injury. Differences between experimental groups were assessed with Chi-Square and Fisher Exact Probability tests.
Quantitative
The goal of these measures was to determine the volume of neocortical damage (VMG) in each of the treatment groups. We corrected for differences in brain size by determining neocortical damage as a percent of the neocortical volume of the left hemisphere (%MG). Finally, we corrected %MG (%MGcorrected) to reflect the only partial representation of neocortex in our frozen material (a methodological limitation inherent to frozen sections; see below). Each brain was coded so that measurements were performed blind with respect to treatment condition.
Cerebral Cortical Volume. Using a drawing tube attached to a Zeiss Universal photomicroscope, the left cerebral cortex was drawn from rostral to caudal pole on serial sections. The distance between the sections (900–1200 µm) was chosen to ensure that at least 7 sections were measured. The cross sectional area of the neocortex was measured from each drawing using NIH Image 1.52 on a Macintosh Centris 650 computer. Total hemispheric volume (VHem) was determined using Cavalieri’s estimation. In rare situations where, because of missing or damaged sections the equi-spaced criteria required for Cavalieri’s estimator was not met, a measurement method involving piece-wise parabolic integration was employed (34).
Microgyric volume. Using a drawing tube attached to a Zeiss Universal photomicroscope, the abnormal area was traced on a series of every 10th section starting from the first section that showed any architectonic distortion and proceeding rostrally until the distortion had unambiguously disappeared. Previous research (17,18) had demonstrated that the appearance of the area of damage with Nissl stains correlated well with assessment of damage as seen with various immunocytochemical stains (i.e., glial fibrillary acidic protein, glutamate, and neurofilament). The cross sectional area of the region of microgyria was measured from each drawing using NIH Image 1.52 on a Macintosh Centris 650 computer. The VMG was determined using Cavalieri’s estimation as described above.
Dependent measures. The percent of damaged cortex (%MG) was computed as %MG = 100 X (VMG/VHem).
In frozen-sectioned brains, the rostral- and caudal-most portion of the cerebral cortex float free from the remaining brain tissue. It is therefore impossible to reliably distinguish left from right hemispheres in these sections. This resulted in only a portion of the entire cerebral being present on each brain, and this portion differed from brain to brain. By underestimating the volume of the cerebral cortex, the value of would be inflated, and the possibility existed that this overestimation was differentially distributed among the experimental groups.
To correct for this, the percent of cerebral cortex that was missing from each of the experimental brains was estimated. As described above, we first estimated the volume of the cerebral cortex on the sections available. We next matched the extent of each frozen-cut brain’s rostral-caudal representation to a complete series of celloidin-embedded material from a previously published study (35). The neocortical volume of the celloidin-embedded tissue was estimated using all the sections from frontal to occipital pole (VµTot). The volume was re-estimated in the same brain based only on those sections corresponding to those sections available in the frozen tissue (VµPart). The ratio of VµPart to VµTot therefore indicated the percent underestimation of VolHem.. The corrected %MG (%MGcorrected) was computed as %MGcorrected = 100 X (VMG/VHem) X (Vµpart/VµTot)
Quantitative data were analyzed with ANOVA procedures with treatment groups as the independent measure and VMG, %MG, and %MGcorrected, as the dependent measures.
Mortality
Nearly 41% (9/22) of the MK2/Les and 61.5% (8/13) of the MK/NoLes (including the one subject injected with 1 mg/kg MK-801) subjects died before P5 (there was no mortality after this point). There was no significant difference in the mortality rates between these two groups (Fisher Exact P > 0.1). In contrast, there was no mortality among the MK1/Les or NoMK/Les treatment groups.
Qualitative
As expected, there was no evidence of cortical damage in any animal in the MK/NoLes (sham-operated) treatment group and they were dropped from the quantitative and qualitative analyses. Those animals from treatment group NoMK/Les (no MK-801 treatment) showed typical microgyria, with the expected four-layered appearance and in all but one case, a microsulcus (see Fig 1 Left). Animals treated with the lower dose of MK-801 (MK1/Les) also showed evidence of microgyria formation, although the depth of that portion of the cortex surrounding the invaginated microsulcus often appeared thinner than in untreated animals (see Fig 1 Middle). In contrast, the type of cerebrocortical malformation found in the animals of treatment group MK2/Les was quite different and far less extensive than in either of the other treatment conditions. In general, the brains of animals treated with the higher dose of MK-801 showed small patches of laminar necrosis and the depth of any invagination, when present, was quite limited (see Fig. 1 Right).
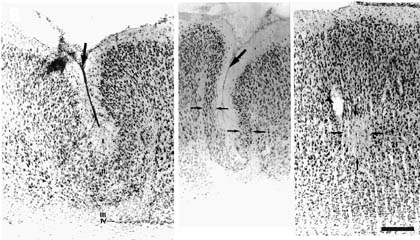 |
| Figure 1. Left. Photomicrograph of microgyric cortex from an animal receiving a freezing lesion but not treated with MK-801 (NoMK/Les). The first layer of the microgyric cortex (layer i) infolds and fuses to produce a microsulcus (large arrow); layer ii, which is continuous with adjacent, normal layers II and III sweeps around the microsulcus; layer iii which is devoid of neurons and contains mostly glia, comprises the lamina dissecans; and layer iv, a thin strip of neurons, is continuous with subplate cells of adjacent cortex. In this section, only the lateral-most portions of layer iv can be seen (small arrow). Middle. Photomicrograph of microgyric cortex from an animal who received a freezing lesion at P1 and who was treated with the lower dose of MK-801 (MK1/Les). Note the formation of a microsulcus (large arrow) and the comparatively thinned layer II (small arrows) when compared to that to the left. Right. Photomicrograph of neocortex from an animal who received a freezing lesion at P1 and who was treated with the higher dose of MK-801(MK2/Les). Note the lack of a microsulcus, with the only damage visible being a relatively small area of necrosis (arrows) in layers IV and V. Arrowhead denotes blood vessel. Bar for all panels = 250 µm. |
Animals were categorized as either having a microsulcus or not. Table 2 summarizes the numbers of animals with and without a microsulcus for each experimental group. Overall Chi-Square analysis demonstrated a significant difference in distribution among the three groups (Chi2 = 13.90, df =2, P < .01). Individual comparisons using the Fisher Exact Probability test demonstrated a difference between MK2/Les and NoMK/Les (P < 0.005), between MK2/Les and MK1/Les (P < 0.05), but not between MK1/Les and NoMK/Les (P > 0.1).
Experimental Group |
Microsulcus |
|
| Yes | No | |
| MK1/Les | 5 | 1 |
| MK2/Les | 3 | 10 |
| NoMK/Les | 11 | 1 |
Presence or absence of microsulcus in the region of induced cerebrocortical malformation.
The distribution is significantly different between MK2/Les and both NoMk/Les and MK1/Les.
Quantitative
The results are summarized in Table 3. Preliminary analysis showed no intraclass correlations between the age of sacrifice and any of the dependent measures. ANOVA demonstrated a significant difference among the treatment groups for all three dependent measures (VMG: F2,28=4.24, P < 0.05; %MG: F2,28=5.49, P < 0.01; %MGcorrected: F2,28=5.13, P < 0.05). Post-hoc analysis for all three measures showed a significant difference between MK2/Les and NoMK/Les (VMG: Mean ± SEM = 2.01 ± 0.58 vs. 5.05 ± 0.89, respectively, Fisher PLSD = 2.15, P < 0.01; %MG: 1.47 ± 0.36 vs. 3.94 ± 0.67, respectively, Fisher PLSD = 1.53, P < 0.01; %MGcorrected: 1.29 ± 0.32 vs. 3.19 ± 0.52, respectively, Fisher PLSD = 1.21, P < 0.01). There were no other significant differences on post-hoc analyses.
Experimental Group |
VMG |
%MG |
%MGcorrected |
| MK1/Les (n=6) | 3.76 ± 1.08 | 2.87 ± 0.78 | 2.24 ± 0.57 |
| MK2/Les (n=13)* | 2.01 ± 0.58 | 1.47 ± 0.36 | 1.29 ± 0.32 |
| MK/NoLes (n=5) | 0 | 0 | 0 |
| NoMK/Les (n=12)* | 5.05 ± 0.89 | 3.94 ± 0.67 | 3.19 ± 0.52 |
*MK2/Les significantly different from NoMK/Les for all three dependent measures, P < 0.05.
In a further analysis of distribution of the measures, a scatterplot of the range of values for each experimental group showed a potential outlier for the NoMK/Les group. When removed from the analysis, the difference among the three groups was still significant for each of the three dependent measures (VMG: F2,27=3.45, P < 0.05; %MG: F2,27=5.24, P < 0.05; %MGcorrected: F2,27=4.59, p < .05).The post-hoc tests similarly revealed a difference between MK2/Les and NoMK/Les (VMG: Mean ± SEM = 2.01 ± 0.58 vs. 4.49 ± 0.75, respectively, Fisher PLSD = 1.99, P < 0.05; %MG: 1.47 ± 0.36 vs. 3.41 ± 0.45, respectively, Fisher PLSD = 1.26, P < 0.01; %MGcorrected: 1.29 ± 0.32 vs. 2.79 ± 0.36, respectively, Fisher PLSD = 1.03, P < .01). Because there was no difference in the analysis among the three dependent measures, we graphically depict only one of them (%MGcorrected) here (Fig 2).
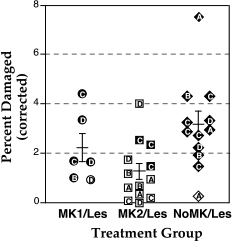 |
Figure 2. Scatterplot of the corrected percent of damaged cortex in treatment groups MK1/Les (circles), MK2/Les (squares), and the control group NoMK/Les (diamonds). Open symbols signify those animals without a microsulcus. Letters within the symbols indicate age of sacrifice: A = P7; B = P10-20; C = P25-60; D = P61 -147. Bars illustrate mean ± SEM for each group. |
DISCUSSION
These results demonstrate that MK-801, when given at a high enough dose, has a significant neuroprotective effect on the formation of microgyria following neonatal freezing injury to the developing neocortex. This effect can be seen both qualitatively and quantitatively. Qualitatively, animals injected with 2 mg/kg MK-801 (MK2/Les) had a less distorted and more focal area of abnormality following freezing injury. Quantitatively, the extent of neocortical abnormality is decreased following injections of MK-801. This is the first demonstration of anatomical neuroprotection in animals at P1—a time occurring before the end of neuronal migration to the cortex is completed. Previously, P7 was the youngest age of rats anatomically assessed for damage in models of neuroprotection from hypoxia and ischemia, and microgyria is not known to occur with lesions at that age.
A number of questions are raised by our results which are addressed below.
Methodological Issues
Mortality
Nearly half of the animals injected with the higher dose of MK-801 (3 X 2 mg/kg) died, irrespective of whether they received freezing injury. All these deaths occurred prior to P5. In contrast, none of the lesioned animals receiving the lower dose (MK1/Les: 3 X 1 mg/kg) died, although the only unlesioned subject to receive the lower dose did. The lower dose of MK-801 was far less effective, however, in ameliorating the effects of the freezing injury than the higher dose. In addition, we have observed (unpublished observations) that fewer injections at the higher dose (one pre-lesion injection followed by one instead of two post-lesion injections at 6 hours), while virtually eliminating mortality, are also less successful in reducing the effect on the freezing injury. The dosages used in this study were at the high end of the range reported in the literature, and these results confirm the relatively high toxicity of MK-801. It is also interesting to note that others have found that while MK-801 has a significant ameliorating effect on the abnormality that follows hypoxic/ischemic damage, the drug does not decrease overall mortality (36). The current results suggest that while the protective effects of MK-801 are encouraging, the use of other, less toxic, neuroprotective agents should be investigated.
The effect of the high mortality rate (41%) in the MK2/Les group on our results should also be considered. It could be, for example, that those animals in this group who had larger than average freezing lesions were more likely to die than those with smaller injury and our measurements in these groups would be, therefore, unnaturally skewed downward. If this were the case, then one would expect that MK-801-treated animals with no lesions would have a significantly decreased mortality in comparison. On the contrary, we found that unlesioned animals who were given the identical dose of MK-801 (treatment group MK/NoLes) had a 61.5% mortality rate. Although it is therefore more likely that mortality in the current study was the result of the toxicity of the pharmacologic treatment, we cannot definitively exclude an interaction between dose and lesion size.
Measurements
Although anatomic assessment of treatment effects of neuroprotective agents is somewhat difficult, the differences seen qualitatively are dramatic. Thus, most of the MK2/Les subjects showed marked changes in the extent and appearance of neocortical damage following the neonatal freezing insult, with only few instances of microsulci present, and focal regions of neuronal loss. All but one animal in the NoMK/Les control group, on the other hand, had large microsulci and showed significant distortions of the cortical laminae (see Fig 1). These differences were also reflected in the quantitative measurements, where a significant decrease in the amount of altered cortex was seen in the MK2/Les group.
We cannot, however, eliminate the possibility that there are other more widespread disturbances following freezing injury, and the effectiveness of neuroprotective treatment on these could not be assessed in the present study. There are suggestions, for example, that injury to the developing cortex results in more widespread disturbances, especially in terms of connectivity. Goldman-Rakic and Rakic (37), for example, found that prenatal frontal or occipital lobe resection resulted in gross changes in the patterns of sulcation in the adult primate brain. Innocenti and Berbel(38,39) have found in the cat that neonatal injection of ibotenic acid in visual cortices, which causes the formation of a microgyric cortex, resulted in the maintenance of connections normally pruned during development. Finally, Miller et al. (40) demonstrated that bilateral carotid occlusion in the developing cat resulted in widespread changes of its callosal connectivity. Whether treatment with neuroprotective agents would affect the connectional reorganization that follows neonatal damage is a question that remains to be addressed.
Early Brain Damage and Neuroprotection
Previous research has shown that MK-801 and other neuroprotective agents effectively ameliorated the anatomic effects of an hypoxic/ischemic insult in rats as young as P7. For example, Trifiletti (41) reported that administration of the potent nitric oxide synthetase inhibitor, NG-nitro-L-arginine 15 hours before hypoxic ischemic insult affords nearly complete neuroprotection. L-kynurenine (42) and kynurenic acid (43) administered to P7 rats after an hypoxic-ischemic event resulted in complete neuroprotection. The calcium channel antagonist, flunarizine, has also been shown to effectively reduce hypoxic/ischemic damage in animals of this age (44). Speiser et al. (13) found that the behavioral effects of P1 anoxia in the rat (hyperactivity and lower performance in passive avoidance) were ameliorated with pre- or post-treatment with MK-801. The current study is the first, however, to demonstrate significant anatomic neuroprotective effects in newborn (P1) animals.
The Etiology of Microgyria and Mechanism of Action of MK-801
We had originally hypothesized that freezing injury to the developing neocortex acts via an hypoxic/ischemic event. Therefore, agents designed to protect the brain from the excitotoxic effects of EAA on NMDA receptors might effectively lessen the effects of the focal injury. There is evidence to suggest, however, that the mechanism of action of MK-801 may lie less in its function as an NMDA antagonist, but rather in its hypothermogenic actions. Thus, the neuroprotective effects of brain hypothermia are well known (c.f., Refs 45,46), and Corbett et al. (7) found that the ameliorative effects of MK-801 are decreased when normothermic brain temperature is maintained. In contrast, however, others have found that the neuroprotective effect of MK-801 is maintained when body or brain temperature is maintained (47-49). The current study was not designed to determine the mechanism of neuroprotection.
Conclusions
Injury may be an important mechanism for the production of developmental malformations in the cerebral cortex. The present study indicates that neuroprotection against damage occurring during neuronal migration may serve to diminish the creation of these neocortical malformations. Future investigations will focus on other, less toxic, neuroprotective agents as well as on the possible amelioration of the more widespread effects of damage to the developing neocortex.
ACKNOWLEDGMENTS
This work was supported, in part, by grant HD20806. The authors wish to acknowledge Judy Richman for technical assistance and Merck & Co., Rahway NJ for supplying MK-801.