Note to the reader: This is a revised edition of a paper published in Brain Research (1995;681:177–189). The definitive original print version is available from Elsevier Press.
New figures, text, and links have been incorporated into the revision. Revised HTML (http://www.nervenet.org/netpapers/Rosen/Radial94/Radial.html) copyright ©1999 by Glenn D. Rosen
BEHAVIORAL CONSEQUENCES OF NEONATAL INJURY OF THE NEOCORTEX
Glenn D. Rosen1, Nicholas S. Waters2, Albert M. Galaburda1, & Victor H. Denenberg2
1Dyslexia Research Laboratory, Beth Israel Deaconess Medical Center; Division of Behavioral Neurology , Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston MA 02215; Harvard Medical School, Boston, MA 02115. 2Biobehavioral Sciences Graduate Degree Program, U-154, University of Connecticut, Storrs, CT 06269-4154.
Address Correspondence to:
Glenn Rosen
Department of Neurology
Beth Israel Deaconess Medical Center
330 Brookline Ave.
Boston, MA 02215
Email:grosen@caregroup.harvard.edu
Several strains of autoimmune mice spontaneously develop molecular layer ectopias that are similar in appearance to those seen in humans and are caused by disturbances in neocortical neuronal migration. These mice also exhibit behavioral anomalies, some of which correlate with ectopias, others with the immunological disorder. In this study, we induced neocortical ectopias (via puncture wounds) and microgyria (via freezing lesions) in the neocortex of one day-old (newborn) mice without immune disorders in an attempt to further disentangle the effects of autoimmunity and of cortical malformation on behavior. In addition, we wished to compare the behavioral effects of small ectopias to larger microgyric lesions.
DBA mice were assigned at birth to receive either a puncture wound or freezing lesion of either the left or right hemisphere. An independent group was subjected to sham surgery. In adulthood, these mice were given a battery of tests designed to measure lateralization and learning capacity. Lesioned mice (irrespective of hemisphere or type of damage) performed poorly when compared to sham-operated animals in discrimination learning, in a spatial Morris Maze Match-to-Sample task, and in a Lashley Type III maze. In shuttlebox avoidance conditioning, where immunological disorder has been shown to compromise behavioral performance in autoimmune mice, there was no difference between lesioned and sham animals. These results (1) support the dissociation between the effects of developmental neocortical anomalies and autoimmune disease on behavior; (2) reveal similarities between spontaneous and induced neocortical malformations; and (3) fail to support a difference in behavioral effects between ectopias and microgyria.
Disturbances of the cerebral cortex during late neuronal migration can result in a variety of minor malformations, including focal dysplasias, subcortical heterotopias, molecular layer ectopias, and focal microgyria (1). These minor malformations have been associated with a wide variety of disorders such as epilepsy (2-4), developmental dyslexia (5-7), callosal agenesis (8), and other, more severe, conditions (9-14). In recent years, animal models have been employed to aid in the characterization of the anatomic and behavioral effects of these anomalies (15-19). For example, molecular layer ectopias are seen in 40% of New Zealand Black (NZB) and BXSB mice (17-19). These ectopias are structurally quite similar to those seen in the humans and are associated with various architectonic and connectional anomalies (20, 21). In the NZB mouse, results from a Mendelian genetic study of ectopias are consistent with a single recessive gene hypothesis (22).
In addition to the anatomic abnormalities, behavioral deficits have been demonstrated in these immune-disordered mice. Mice with ectopias performed more poorly in the Morris Maze, a task strongly dependent on spatial cues (23), thereby suggesting that these minor neocortical malformations have a direct effect on behavior. Further, left-pawed ectopics performed better than right pawed ectopics in learning a water escape task in male and female NZB and male BXSB mice (24). In addition, NZB mice are poor active avoidance learners, irrespective of whether they have ectopias or not (25-27).
There remains a potential confound because of the simultaneous presence of ectopias and autoimmune disease in the same animal. It could be, for example, that these ectopias interact with immune disorders to produce the behavioral anomalies. Previous research has shown, however, that deficits in active avoidance conditioning are related solely to the presence of auto-antibodies and not to ectopias (27-30). Conversely, association learning is impaired in NZB with ectopias only, irrespective of immune status. This double dissociation supports the idea of separate effects of autoimmunity and abnormal anatomy. Another way to confirm this dissociation would be by inducing equivalent malformations in immunologically normal animals, which is the focus of this report.
Using puncture wounds placed in the first days of life, we have successfully induced molecular layer ectopias (that are similar to those occurring spontaneously in the NZB and BXSB mouse) in otherwise normal mouse neocortex (31,32). Focal four-layered microgyria can be induced by freezing lesions to the neocortex placed during the same time frame (33-38). In the current experiment, we induced anatomical anomalies (ectopias and microgyria) in the brains of otherwise normal mice of the DBA strain to determine the effects of developmental neuropathologic lesions on behavior directly without the potential confounds of autoimmunity.
METHODS
Subjects
A total of 116 DBA mice born at the animal facility of Beth Israel Hospital from DBA/2J foundation stock obtained from The Jackson Laboratory (Bar Harbor, ME) were used.
Protocol
On the day of birth (P0) or P1, pups within a litter were randomly assigned to receive either a puncture wound, a freezing lesion, or sham surgery. For each surgical condition, pups randomly received a lesion to either the right or left hemisphere. After surgery, the animals were returned to their mothers and were left undisturbed until weaning (P25-30). Around P45, mice were transported to the Developmental Psychobiology Laboratory of the University of Connecticut for behavioral testing. After completion of testing, animals were perfused transcardially with 10% formalin, their heads removed and shipped to back Beth Israel Deaconess Medical Center for histological processing.
Induction of neocortical anomalies
Ectopias
The procedure for inducing neocortical ectopias is modified from methods previously described (32). Under hypothermia-induced anesthesia, a 25 gauge hypodermic needle was manually inserted through the scalp and the skull into the underlying cerebral cortex of either the left or the right hemisphere. Two puncture wounds were performed for each animal, taking care to place them at a distance sufficient to avoid confusion on subsequent histology. Two wounds were placed in order to insure that at least one was successful. Following the puncture wounds, subjects were uniquely marked with ink injections to the footpads, the wound was sealed with NuSkin (Medtech Laboratories, Jackson, WY), and the pups warmed under a heat lamp and returned to their mothers.
Microgyria
Focal necrotic lesions were induced in animals based on a modification of the technique employed by Dvorák and colleagues (8,9) and reported in detail elsewhere (33,34). Briefly, pups were anesthetized with hypothermia, and a small incision was made in the antero-posterior plane of the skin over the cerebral hemisphere assigned to receive the lesion. A cooled (~ -70°C) 1 mm diameter stainless steel probe was placed on the skull of lesion subjects, approximately midway between bregma and lambda, for 5 seconds. After placement of the probe the skin was quickly sutured and the wound sealed with NuSkin. The subjects were uniquely marked with ink injections to the footpads, warmed under a lamp and returned to the mother.
Sham Surgery
Animals receiving a sham surgery were treated identically to those receiving puncture wounds except that the needle did not pierce the scalp.
Behavior Testing
Paw Preference and Paw Asymmetry
Collins test. The mouse was food deprived (water ad libitum) and then placed into a small cubicle containing a cylindrical feeding tube projecting at chest height when the mouse stood on its hind legs (39,40). The mouse reached for grains of sweetened rolled wheat (Maypo) inside the tube and was observed until 50 paw reaches had been made. The paw preference score was the number of right paw entries (RPE). If the animal quit before 50 reaches, the score was adjusted appropriately (e.g., 22 RPEs in 35 attempts was adjusted to a score of 31). The RPE score combined both direction and degree of asymmetry. Paw asymmetry was measured by subtracting 25 from each RPE score and dropping the algebraic sign. Thus mice with RPE scores of 0 and 50 would have the same asymmetry score of 25.
Lateral Paw Preference. A measure of lateral paw preference (LPP) was obtained as described in detail elsewhere (41). Briefly, the test was similar to the Collins test described above with the exception that the preferred food was available ad libitum in two containers within the LPP apparatus. The amount of food consumed from the right and left hoppers was recorded over an 8-10 hour period over 5 consecutive days. This test was repeated twice, and the combined laterality index (LI) was computed as: where R was the amount of food consumed in the right hopper and L was the amount consumed in the left.
Swimming Rotation
This test has been described in detail (42). Briefly, the mouse was placed in a cylinder (30.5 cm diameter) partially filled with water (21-22°C) and allowed to swim for 5 min. The mouse's movements were tracked by an observer using a joy stick attached to a computer. The computer recorded number of quarter turns clockwise (CW) and counterclockwise (CCW). Three scores were then derived: (1) quarter turn laterality index (QTLI), defined as (2) the magnitude of asymmetry, independent of direction, obtained by taking the absolute value of QTLI (|QTLI|); and (3) Sum-QT, the sum of CW and CCW turns, which was used as an index of activity.
Water Escape Learning
The mouse was put into an oval tub (51.2 X 102.4 cm) partially filled with water (17–19°C) and had to swim to a submerged platform at the far end. It was allowed 120 sec to find the platform. If found, the animal remained on the platform for 20 sec and was then placed into a mouse box with shavings under a heat lamp. If it did not find the platform, it was guided there, left for 20 sec, and then put in the heated mouse box. The other animals in the squad were given their first trial in the same manner. All animals were then given their second trial. This continued for five trials. The score was time to reach the platform.
Multiple Activity Measures
The Omnitech Digiscan Animal Activity Monitor was used. This unit has a series of infrared photocells at right angles to each other at floor level (X and Y axes), and other photocells at a higher level which were activated when the animal reared up (the Z axis). The Z-axis recorder was not used in these experiments. A mouse was placed into a square chamber, 20 cm per side and 30 cm high, for one 15-min session, and activity records were obtained for each 5-min segment. The two measures reported are horizontal and vertical activity.
Shuttlebox Avoidance Learning
The Omnitech Shuttle-Scan Model SCII system was used. A partition with a hole at floor level separated the box into two compartments. Foot shock (0.4 mA) was delivered through an electronic scrambler. The animal's location was sensed via infrared beams located in each compartment. The CS was a pulsating light, and the CS interval was 5 sec. When shock occurred, the CS remained on. If the animal ran into the opposite compartment, both shock and light went off, and the intertrial interval (ITI) started. If the mouse did not cross to the other compartment after 20 sec of shock, both shock and light went off and the ITI started. The ITI was randomly distributed around 20 (± 5) sec. During the ITI the mouse could move freely between compartments.
Each trial was classified as an avoidance response (crossing to the other side before the onset of shock), an escape response (crossing to the other side after shock occurred), or a null response (remaining in the original compartment and receiving 20 sec of shock). In addition, time to avoid was recorded for the avoidance trials, and time to escape was recorded for the escape trials.
At the start of each day's testing the animal was given a 5 min adaptation period in the apparatus and could move about freely. Each mouse was given 50 trials a day for 5 days.
Discrimination Learning
The apparatus was a two-arm swimming T-maze (water temperature 17-19°C). The stem was painted grey, one alley was black and the other white. The alleys were curved back toward the stem so the mouse could not see the wire mesh escape ladder hung at the end of the positive alley (43). The computer program for tracking and scoring an animal has been described (44). Each animal was randomly assigned black or white as the positive stimulus. The left-right location of the positive stimulus for each day's 10 trials was determined by selecting a semi-random sequence. The mouse was placed into the water at the stem end and allowed 60 sec to find the ladder. After arriving at the escape ladder, the mouse was removed and put into a dry mouse box under a heat lamp where it remained until all members of the squad had been tested. It was then given its second trial. Ten trials were given per day for five days. One measure of learning was the number of correct responses, defined as swimming down the stem of the T and making a correct choice without entering the incorrect alley. The second measure was the median time needed to complete the 10 trials within a day. Finally a Discrimination Learning Score was computed (see Ref 44 for details).
Lashley III Maze
This was a relatively complex maze which contains cul-de-sacs that the animal has to learn to avoid, and T-choices where the animal has to learn to make the correct left or right turn. We used a water version of the maze, with the temperature at 21–22°C. As the mouse swam through the maze, its path was typed into a computer where a program analyzed the data into correct entries and errors. The apparatus and scoring procedure has been described in detail (24). The mice were given two trials a day (one in the morning and one in the afternoon) for five days. The two daily trials were pooled, yielding 5 trial blocks. The maze can be learned by use of extra-maze spatial cues or by memorizing the chain of correct left and right turns. Three measures of learning were obtained: a learning index defined as number of correct entries divided by total number of entries, total number of daily forward errors, and total number of daily backward errors.
Morris Maze Match-to-Sample
This test utilized the Morris maze procedure, as adapted to spatial delayed-matching-to-sample (45,46). In this task, the animal is given four trials with the platform in the same location. This constitutes a "problem." For the next four trials, the platform is randomly moved to a different location. This constitutes a second problem. Subjects received 2 problems of 4 trials per day for 5 days, with approximately 2 hours between the problems on each day.
For each trial, each mouse was placed in a tub of water at any of four locations, and had to find a submerged platform. We used a circular black tub (123 cm diameter), with water 18-21°C. The platform (8 cm diameter) was set 40 cm from the wall. As the subject swam from the start position, its path was traced on an electronic digitizing tablet containing a template of the maze (44). Each mouse was given four trials (maximum of 45 s/trial) per problem, one from each of the four start positions in a pseudo-random order. The escape platform remained in the same position throughout the problem. After each trial, the subject remained on the platform for 10 sec. The animals were tested in squads of 5 or 6 mice. Within each squad, all animals received trial 1, then all received trial 2, etc. If subjects did not attempt to escape, that is, they floated motionlessly, they were given a tail pinch with forceps. Subjects were returned to their home cages and placed under warming lights between trials.
Anatomical analysis of brains
Perfusions were performed at 14-30 weeks of age. The brains were removed from the skulls and placed in formalin for at least one week. They were then dehydrated in 80%, 95%, 100% ethanol and ethanol/ether. The brains were embedded in 3% celloidin for 3-4 days followed by 12% celloidin for 2–3 days or until hard. Afterwards they were cut into gapless 30µm coronal sections, and every fifth section was stained with cresyl violet for Nissl bodies and mounted on glass slides. The slides were examined under the light microscope by an experimenter who was blind as to their condition and the presence of cortical ectopias, dysplasias, microgyrias, and other types of brain abnormalities was noted.
In those brains exhibiting cerebral cortical malformations, the location and extent of damage was quantified. Using a drawing tube attached to a Zeiss Universal photomicroscope, the abnormal area was traced on a series of every 10th section starting from the first section that showed any architectonic distortion and proceeding rostrally until the distortion had unambiguously disappeared. The cross sectional area of the lesion was measured from each drawing using NIH Image 1.52 on a Macintosh Centris 650 computer. The total volume of the lesion (Vlesion ) was determined using Cavalieri’s estimation (47). In the case of the puncture wounds, where there were two distinct areas of damage, the volume was computed as a sum of both the rostral and caudal regions of damage. The architectonic location of the lesion was also quantified by overlaying the topographic location on a normalized flattened map of the neocortex derived from Zilles (48).
In addition to measuring Vlesion, an estimate of cerebral cortical volume was made in a subset (10) of the lesioned subjects. Using a drawing tube attached to a Zeiss Universal photomicroscope, each cerebral cortical hemisphere was drawn from rostral to caudal pole on a series of every 20th section. The cross sectional area of the neocortex was measured as described above and the total hemispheric volume was determined using Cavalieri’s estimation. The percent of cortical damage (%Vlesion ) was computed by dividing by the average of the cerebral cortical hemisphere volume (14.3 µm3).
RESULTS
Anatomical Analysis
Of the 116 brains examined, four with purported unilateral freezing lesions had lesions close to the midline which resulted in bilateral damage. In addition, two other animals with freezing lesions developed porencephalic cysts and hydrocephalus. All six were removed from the analysis.
Histological examination of the brains of the lesioned mice confirmed that the gross appearance of puncture wounds is different from that of freezing lesions (Fig. 1). Specifically, animals with puncture wounds generally had at least one (N=7), and more often two (N=38), focal areas of disturbance with nests of ectopic neurons in layer I of the neocortex. Freezing injury on the other hand generally led to more severe disturbance along with the formation of microgyria. Although the two types (puncture and freezing) of lesions were distinct from each other, the disturbance associated with the freezing injury in the mouse was far less severe than that associated with a similar injury to the rat neocortex (33,34). In the rat, freezing injury results in microgyria with microsulcus formation greater than 95% of the time. In contrast, only about 25% of the mice with freezing lesions had a microsulcus in this study.
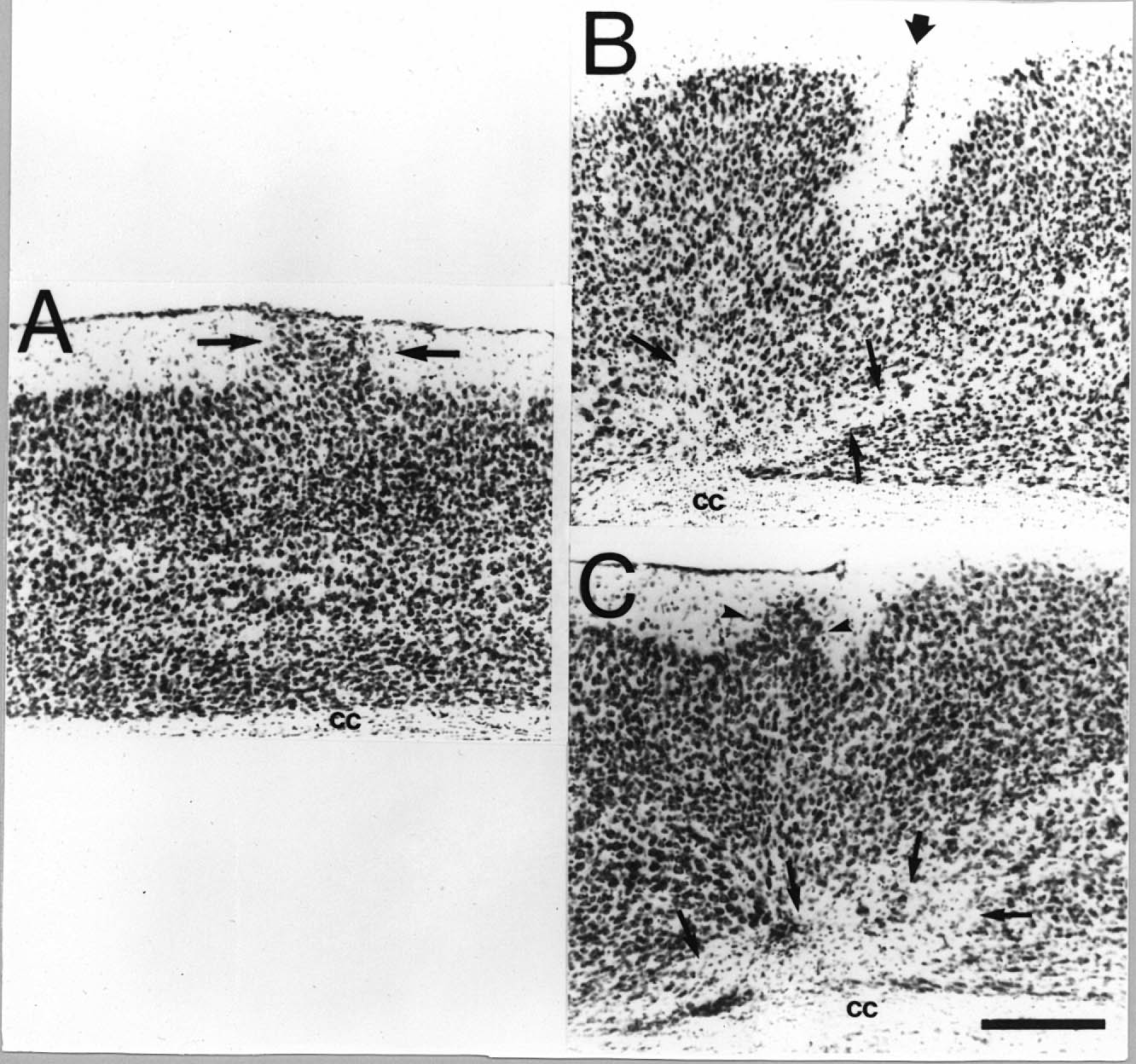 |
Figure 1. Comparison of puncture wounds and freezing lesions. A. Molecular layer ectopia (arrows) in an adult DBA mouse induced by a puncture wound on P1. B. Microgyria in an adult DBA mouse induced by a freezing injury on P1. Note microsulcus (large arrow) near the pial surface and cell free areas in the subgranular layers (small arrows). C. Area of microdysgenesis in an adult DBA mouse induced by a freezing injury on P1. Note bulge of neurons from layer II into the molecular layer (arrowheads) which, while similar to that seen in panel A, is mainly differentiated by the lack of neurons reaching the pial surface. Note that although there is a lack of a microsulcus as seen in panel B, there is a similar cell free area which is characteristic of this type of lesion (arrows). Bar for all panels = 200 µm. cc = corpus callosum.
[click here or on figure for enlargement (236K)] |
The location of the lesions are illustrated in Fig. 2. Freezing lesions centered around the frontal and somatosensory regions of the neocortex, including the hindlimb and forelimb areas. The lesions in the right hemisphere were more medially situated than those of the left hemisphere, with more frontal damage in the former. The rostral puncture wounds were also centered around the forelimb portion of the somatosensory cortex while the caudal puncture wounds were predominantly in the visual cortices. The location of the caudal lesions was slightly more rostral in the left hemisphere as compare to its right counterpart. The means for Vlesion and %Vlesion are also seen in Fig. 2. A two-way ANOVA with Hemisphere (right or left) and Lesion Type (Puncture or Freezing) showed a significant main effect of Lesion Type for both dependent measures, with the microgyric animals having larger areas of damage than those with puncture wounds (F1,75 = 48.04, P < 0.001). There was no difference between the volume of damage between the left and right for either dependent measure (F1,75 = 2.67, ns).
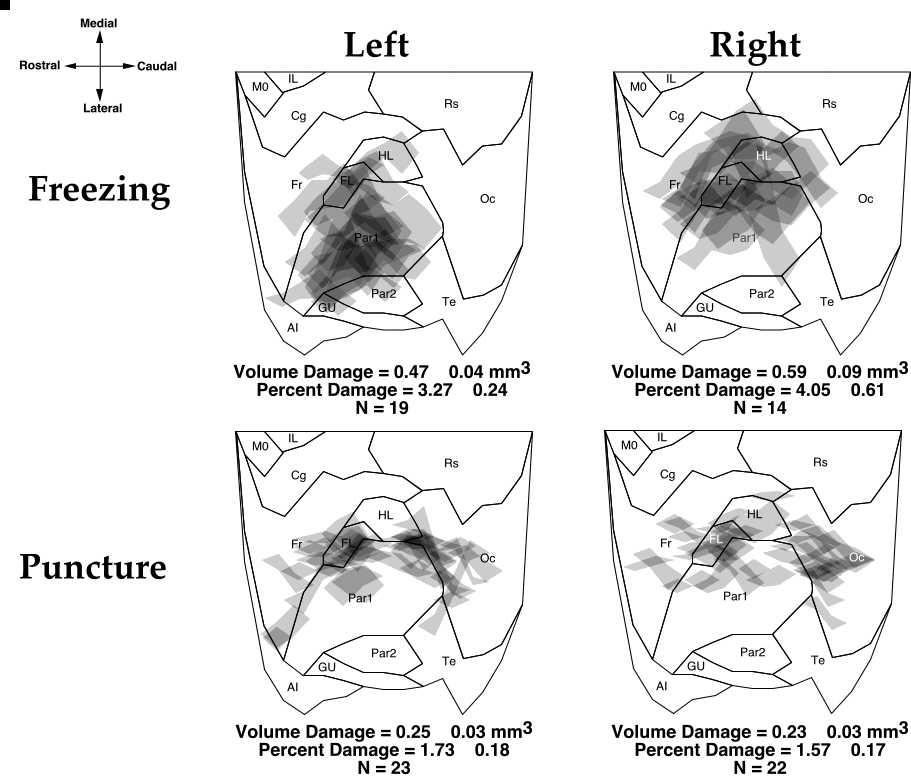 |
Figure 2. Topographic location of freezing lesions and puncture wounds from the left and right hemispheres of mice placed over a flattened, normalized map of the neocortex derived from Zilles (48). Each lesion is plotted and areas of overlap are indicated by progressively darker shades of gray. The means ± S.E.M. for the volume and percent damage are found below each map. Abbreviations: AI - agranular insular (includes dorsal, posterior, and ventral part); Cg - cingulate cortex (included Cg1-3); FL - forelimb area; Fr - frontal cortex (includes areas Fr11, Fr2, and Fr3 ); Gu - gustatory cortex; HL - hindlimb area; IL - infralimbic area of the medial frontal cortex; MO - medial orbital area; Oc - occipital cortex (includes all subdivisions of Oc1 and Oc2); Par1 and Par2 - primary and secondary somatosensory cortices, respectively; RS - retrosplenial cortex (includes granular and agranular subdivisions); Te- primary auditory cortex, and temporal areas 2 and 3.
[click here or on figure for enlargement (268K)] |
Preliminary Statistical Analysis
During the course of behavioral testing 5 animals died and were removed from the analysis. In addition, four animals were discovered to be pregnant due to late weaning and were not tested. Counting the 6 eliminated for anatomic reasons, we were left with 101 animals (55 male and 46 female) for analysis.
For each of the dependent measures, we performed a 2-way ANOVA with Type of Lesion (puncture or freezing) and Hemisphere as the independent measures. Despite finding significant differences in the degree of anatomic damage between freezing and puncture wounds, as well as differences in location between the hemispheres, we found no significant main effects of either independent measure, nor any significant interactions with any of the dependent measures. We therefore pooled over these conditions and, for the analyses that follow, the independent measures will be Lesion (lesioned or sham) and Sex. For the learning tests, Days (or Trials) is included as a repeated measure.
Behavioral Analysis
Not all mice received the entire test battery. The number of subjects tested and the results are summarized in Table 1.
Paw Preference
Collins test. There were no significant effects of Lesion or Sex, nor were there any significant interactive effects.
LPP. There was a significant difference in LI with lesioned animals being more rightward biased than unlesioned animals (F1,85 = 3.98, P < 0.05). When the 11 animals who subsequently developed seizures in the discrimination learning apparatus (see below) were removed from this analysis, however, the difference was no longer significant (F1,74 = 2.62, ns). There were no main effects of Sex nor were there any interactions.
Swimming Rotation
There were no main effects for Lesion or Sex, although there was a significant interaction for |QTLI|(F1,97 = 4.69, P < 0.05) with unlesioned females having a greater rotational asymmetry than any of the other three groups (see Fig. 3).
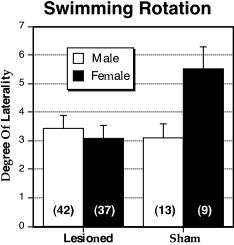 |
Figure 3. Mean ± S.E.M. of |QTLI| in the swimming rotation apparatus for male (white bars) and female (black bars), lesioned and sham DBA mice. Female shams have greater absolute laterality than the other three groups. N’s in parentheses. |
Water Escape Learning
There were no main effects for Lesion or Sex, nor was there a significant interaction. There was a significant Days effect, indicating that learning occurred over the five days of testing (F4,388 = 20.4, P < 0.001).
Multiple Activity Measures
There was a significant main effect of Sex for total horizontal movements in the Digiscan apparatus (F1,97 = 4.29, P < 0.05) with males being more active than females. There were no significant differences for vertical movements.
Discrimination Learning
The decrease in the number of mice available for this and subsequent tests was due to 11 males (9 lesioned and 2 sham) developing seizures in this apparatus. Removing these animals from the analyses for the previous tests did not changed only the results for LPP where the lesioned/sham difference was no longer significant (see above). There were significant main effects for Lesion and Days which are summarized in Fig 4. Sham animals performed better than lesioned animals in the total number of correct choices (F1,86 = 5.42, P < 0.05) and discrimination learning score (F1,86 = 7.63, P < .01). There was a significant Day X Sex interaction for both total correct choices (F4,344 = 3.42, P < .001) and learning score (F4,344 = 2.66, P < 0.05), with females performing better on day 5 than males (see Fig. 4C, D). All animals showed significant learning over days on all dependent measures (P < 0.001 in all cases).
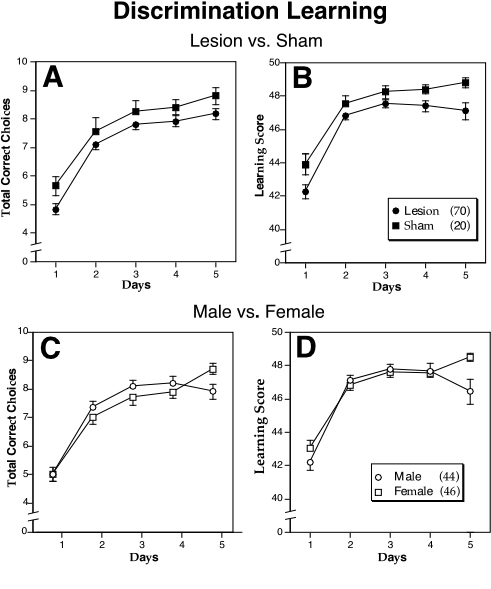 |
Figure 4. The mean number (± S.E.M.) of total correct choices (A, C) and learning score (B,D) in the discrimination learning apparatus for lesioned (closed circles) vs. sham (closed squares) or male (open circles) vs. female (open squares) mice. In cases where there is overlap of the S.E.M., only the positive or negative values are shown. N’s in parentheses in the legend.
[click here or on figure for enlargement (96K)] |
Shuttlebox Avoidance Learning
Females avoided more than males in this apparatus (F1,95 = 4.96, P < 0.05), and in a complementary manner, males escaped more than females (F1,95 = 5.11, P < 0.05). For avoidance time, a significant Day X Sex interaction was found (F4,368 = 2.78, P < 0.05), with females decreasing their time more rapidly than males (Fig. 5). There was a significant Day effect for all five dependent measures (P < 0.001 in all cases). There was no effect demonstrated for Lesion.
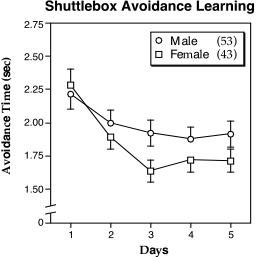 |
Figure 5. Mean (± S.E.M.) shuttlebox avoidance time over 5 days for male (circles) and female (squares) mice illustrating the Trial ¥ Sex interaction. Females improve their avoidance time over days in comparison to males. In cases where there is overlap of the S.E.M., only the positive or negative values are shown. N’s in parentheses in the legend. |
Lashley III Maze
The Lesion ¥ Sex interaction for the learning index was significant (F1,85 = 5.66, P < 0.05) with sham males having a higher LI than the other three groups (Fig. 6). This ANOVA also showed that the main effect for Lesion approached significance (Fig 7A-C) with shams making less forward and backward errors that lesioned mice (F1,85 = 3.39, P = 0.069; F1,85 = 3.48, P = 0.066, respectively). Learning occurred over the five days for all measures (P < 0.001 in all cases).
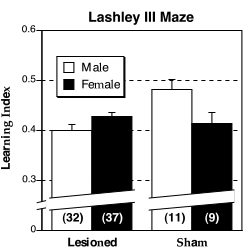 |
Figure 6. Mean (± S.E.M.) learning index on the Lashley III maze for male (white bars) and female (dark bars) DBA mice. Male shams have a higher learning index than the other three groups. N’s in parentheses. |
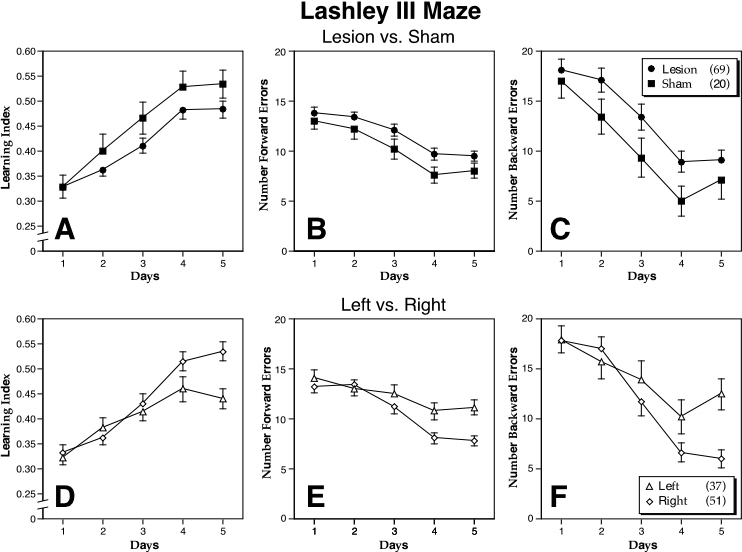 |
Figure 7. Mean (± S.E.M.) of three dependent measures of Lashley III maze performance over 5 days of testing. These include the learning index (A,D) and the number of forward (B,E) and backward (C,F) errors. Panels A-C compare lesioned (closed circles) vs. sham (closed squares) DBA mice, with shams performing better than lesioned animals. Panels D-F compare left- (triangles) vs. right-pawed (diamonds) DBA mice, with right-pawed animals performing better than their left-lesioned counterparts. In cases where there is overlap of the S.E.M., only the positive or negative values are shown. N’s in parentheses in the legend.
[click here or on figure for enlargement (128K)] |
Morris Maze Match-to-Sample
There were significant main effects for Lesion (F1,57 = 5.61, P < 0.05) and Sex (F1,57 = 7.27, P < 0.01) for distance, but not time (Fig. 8). Lesioned animals and females traveled longer distances across the 10 problems. There were significant Trial and Block effects for both distance and time (P < 0.001 in all cases). There were no significant within-problem effects (see Discussion).
 |
Figure 8. Mean (± S.E.M.) of distance traveled in the Morris Maze Match-to-Sample test for male (white bars) and female (dark bars) DBA mice. Lesioned mice perform worse than shams and females perform worse than males. N’s in parentheses. |
Analysis After Categorization by Lateral Paw Preference
Because previous research had found that paw preference (Collins test) significantly interacted with ectopia (24), we categorized animals as either right- (26 or more RPE) or left-biased (25 or less RPE) and analyzed the data in a 2-way ANOVA using Lesion as the other independent measure.
There were no significant main effects or interactions with paw preference for any of the dependent measures with the exception of the Lashley Maze. For all three dependent measures in this task, there was a significant effect of Lesion with shams performing better than their lesioned counterparts (P <0.05 in all cases). There was also a Day X Paw Preference interaction for all dependent measures (P < 0.01 in all cases) with right-pawed animals performing better during the last days of testing than left-pawed animals (Fig. 7D-E).
DISCUSSION
These results demonstrate that neonatally induced neocortical malformations can significantly affect behavior. Specifically, we found that sham mice performed better than lesioned mice in discrimination learning and took shorter paths to the target in the Morris Maze Match-to-Sample task. When paw preference was considered as an independent measure, shams were found to make fewer errors in the Lashley III maze and right-pawed mice had faster learning rates than left pawed. irrespective of lesion group. In addition, there were three sex effects in this study: (1) males were more active than females in the horizontal plane of the Digiscan apparatus, (2) males traveled shorter distances than did females in the Morris Maze Match-to-Sample paradigm, and (3) females avoided more and escaped less in the active avoidance shuttlebox test. The latter results confirm previously published reports in which females of other strains of mice have been shown to perform better in active avoidance than males (28).
It is important to note that all subjects showed evidence of learning in all tests. Thus, there was never an example where one group (male/female; lesioned/sham) learned the task while the other did not. This and other similarities between the behavioral effects of induced and spontaneous neocortical malformations as well as some differences are discussed below.
Behavioral Effects of Spontaneous and Induced Neocortical Malformations
Previously, we reported that NZB mice with ectopias take longer to escape at the ladder in the discrimination learning apparatus than do non-ectopic animals (23), Interestingly, this deficit is ameliorated by placing ectopic animals into enriched environments. Additionally, ectopic mice of the BXSB-Yaa+ strain perform worse than non-ectopic mice on this task (unpublished observations). In the current report, lesioned animals were deficient in discrimination learning, thereby supporting the notion that a deficit in performance in this task is related to developmental neocortical anomalies.
Previous research has shown no association between performance in the active avoidance paradigm and the presence of spontaneous ectopias (23, 27-29). There is, however, a relationship between immunological status and behavior on this task, whereby the greater the immune-disorder the worse the performance (27-30). In addition, mice reared in the uteruses of immune-disordered mothers perform poorly, irrespective of their genetic history (27). In the current study, we found no difference between lesioned and sham animals in avoidance learning, thereby supporting the hypothesized dissociation between the effects of developmental neocortical malformations and immune disorders on behavior. In order to make this dissociation complete, however, it would be necessary to show that mice with induced malformation did not develop autoimmune disease as a result of the surgery. While we did not immunologically test the animals in this study, we have found that DBA mice with induced ectopias do not differ from their unlesioned counterparts on a variety of tests of immune function, including measures of cardiolipin and DNA antibody concentration (S. Ansar Ahmed, unpublished observations).
Although there are similarities between the results reported here and those from behavioral studies of mice with spontaneous neocortical anomalies, there were some differences as well. We had previously reported, for example, that left-pawed ectopic mice performed better than their right-pawed counterparts in water escape. In the current study, however, we found neither an interaction between pawedness and ectopia, nor did we find any main effect for this task. In the case of water escape, therefore, there is a lack of coherence between the effects of spontaneous and induced neocortical malformations on behavior.
In the Morris Maze Match-to-Sample task, we found no differences between lesioned and sham animals on within-problem performance (a measure of working memory), though ectopias affect performance on this measure in BXSB mice (46). In contrast, we found in the current study that lesions produced a deficit in across-trial performance, indicating differences in reference memory or in general spatial ability. This has also been found in NZB mice (23).
Finally, NZB mice in general exhibit poor performance on the Lashley III maze in comparison to BXSB and other strains, and there is no relationship between the presence of ectopias and this behavior in either strain (24, 49). The DBA mice in the current study performed better than NZB mice, but lesioned animals made more errors than their unlesioned counterparts. More specifically, male shams performed better than males and females of the lesioned group as well as female shams.
Developmental Neocortical Malformations
The mechanism by which these small, focal neocortical anomalies affect behavior remains unknown. Neonatal damage to developing neural structures leads to profound and sometimes pervasive reorganization of the brain (50-53). While others have shown that lesions to the developing cortex can have profound effects on behavior, these lesions are far larger than those reported here (54,55). We have hypothesized that the focal developmental neocortical anomalies that are seen spontaneously in immune-disordered mice are associated with disturbance of connectivity (20). We have seen, for example, changes in the pattern of callosal connectivity associated with a region of microgyria, as well as more pervasive disturbances (56). In addition, regions of abnormal neurofilamentous bundles have been seen in spontaneously occurring ectopias of NZB mice (20). Innocenti and Berbel (57,58) have found in the cat that neonatal injection of ibotenic acid in visual cortices, which causes the formation of a microgyric cortex, resulted in the maintenance of auditory connections normally pruned during development. Miller et al. (59) demonstrated that bilateral carotid occlusion in the developing cat resulted in widespread changes of its callosal connectivity. These findings support the notion that early focal neocortical damage of the type reported here may have profound and pervasive disturbances in cortical connectivity.
As detailed above, there are numerous reports of the effects of spontaneous ectopias on behavior. We have recently reported that microgyria in the barrel field region in the rat can profoundly disrupt auditory discrimination learning in the rat (60). Specifically, animals with bilateral microgyria, as compared to unoperated controls, have difficulty in performing fast, but not slow, auditory discrimination. This is true even though the area of damage does not extend into auditory cortex, suggesting again that the hypothesized widespread changes in connectivity associated with these types of lesions may have wide-ranging behavioral effects. Relatedly, it is interesting to note that in the current study we could find no behavioral differences between the types of lesion—freezing or puncture—despite the fact the former resulted in a larger percentage of cortical damage than the latter. In addition, the difference in lesion localization between the hemispheres also did not result in any behavioral differences. This may be due to the aforementioned connectional changes that are associated with both types of injury. It is possible that a ceiling effect is quickly reached by a neonatal lesion in its ability to release trophic factors that support massive reorganization. Thus, whether a lesion produces ectopias or microgyri may be less important to behavioral outcome than whether the timing of the insult may have had an adverse effect on the connectional organization of the brain and, subsequently, behavior.
Summary and Conclusions
Previous research demonstrated that autoimmune mice with spontaneous neocortical ectopias were deficient in the discrimination learning paradigm. Further, although there were no differences between ectopics and non-ectopics in shuttlebox avoidance learning, immunological disorder compromised the ability to perform. It was hypothesized that the two pathological components of the NZB and BXSB genotype—neuroanatomic and immunologic—had dissociable effects on behavior. We report here that immunologically normal mice with induced neocortical malformations were deficient in discrimination learning, the Lashley III maze, and a Morris Maze Match-to-Sample task. There were no differences between lesioned and sham animals in shuttlebox avoidance learning, however. These findings support the hypothesized dissociation between anatomic and immunologic effects on behavior. Further these results demonstrate again that induced focal neocortical malformations can adversely affect behavior. Finally, the lack of a difference on behavior of the two types of malformations as well as the lack of association with location, points to the relative importance of timing of injury as opposed to the degree or the location of the damage.
ACKNOWLEDGMENTS
The authors thank Dr. Gordon F. Sherman for his careful review of the manuscript and the referees for their helpful suggestions. We would also like to thank Alison R. Frank, Antis Zalkalns, and Nancy Talgo for technical assistance. Thanks goes also to Noel Gouveia for his help with the graphics. This work was supported, in part, by PHS Grant HD 20806.
Table 1
Summary of ANOVA results for all behavioral measures
**Males differ significantly from Females
Dependent measure |
Lesioned |
Sham |
Male |
Female |
| Paw Preference | ||||
| RPE | 26.81 ± 1.21 | 24.96 ± 2.42 | 26.82 ± 1.42 | 25.89 ± 1.68 |
| Paw Asymmetry | 8.89 ± 0.70 | 8.41 ± 1.57 | 8.47 ± 0.86 | 9.16 ± 0.97 |
| N | 78 | 22 | 55 | 45 |
| LPP LI* | 0.41 ± 0.14 | -0.17 ± 0.27 | 0.20 ± 0.18 | 0.39 ± 0.18 |
| N | 70 | 19 | 49 | 40 |
| Swimming Rotation | ||||
| QTLI | -0.15 ± 0.48 | 0.33 ± 1.02 | -0.30 ± 0.57 | 0.25 ± 0.67 |
| |QTLI| | 3.26 ± 0.31 | 4.08 ± 0.5 | 3.34 ± 0.35 | 3.55 ± 0.41 |
| Sum-QT | 141.2 ± 5.28 | 141.9 ± 7.33 | 146.0 ± 5.71 | 135.8 ± 6.85 |
| N | 79 | 22 | 55 | 46 |
| Digiscan | ||||
| Horizontal* | 3858.1 ± 104.9 | 3776.8 ± 249.0 | 3996.2 ± 131.6 | 3654.1 ± 145.5 |
| Vertical | 277.9 ± 45.14 | 396.0 ± 121.6 | 251.4 ± 34.4 | 366.2 ± 87.36 |
| N | 79 | 22 | 55 | 46 |
| Water Escape | 72.32 ± 2.35 | 61.34 ± 4.71 | 66.28 ± 2.84 | 74.30 ± 3.14 |
| N | 79 | 22 | 55 | 46 |
| Discrimination Learning | ||||
| Total Correct Choices* | 7.16 ± 0.10 | 7.73 ± 0.19 | 7.31 ± 0.13 | 7.26 ± 0.13 |
| Learning Score* | 46.23 ± 0.19 | 47.38 ± 0.26 | 46.25 ± 0.26 | 46.71 ± 0.19 |
| Time to Escape | 7.31 ± 0.41 | 5.39 ± 0.49 | 7.84 ± 0.58 | 5.98 ± 0.34 |
| N | 70 | 20 | 44 | 46 |
| Shuttlebox Avoidance | ||||
| Avoidance** | 37.35 ± 0.63 | 37.00 ± 1.30 | 35.99 ± 0.77 | 38.89 ± 0.84 |
| Escapes** | 12.49 ± 0.62 | 12.87 ± 1.27 | 13.84 ± 0.75 | 10.99 ± 0.83 |
| Null | 0.11 ± 0.05 | 0.12 ± 0.08 | 0.10 ± 0.04 | 0.12 ± 0.08 |
| N | 78 | 21 | 55 | 44 |
| Avoidance Time | 1.91 ± 0.03 | 1.96 ± 0.04 | 1.98 ± 0.03 | 1.85 ± 0.03 |
| N | 76 | 20 | 53 | 43 |
| Escape Time | 5.85 ± 0.04 | 5.75 ± 0.06 | 5.80 ± 0.04 | 5.87 ± 0.05 |
| N | 71 | 20 | 55 | 36 |
| Lashley (over five days) | ||||
| Learning Index | 0.42 ± 0.01 | 0.45 ± 0.02 | 0.42 ± 0.01 | 0.42 ± 0.01 |
| Forward Errors | 11.71 ± 0.26 | 10.21 ± 0.45 | 10.95 ± 0.31 | 11.77 ± 0.34 |
| Backward Errors | 13.34 ± 0.54 | 10.40 ± 0.89 | 12.68 ± 0.63 | 12.67 ± 0.69 |
| N | 69 | 20 | 43 | 46 |
| Morris Maze Match-to-Sample | ||||
| Time | 36.04 ± 0.35 | 34.36 ± 0.65 | 36.05 ± 0.46 | 35.29 ± 0.42 |
| Distance* | 346.1 ± 3.49 | 311.2 ± 5.95 | 315.9 ± 4.26 | 354.6 ± 4.19 |
| N | 46 | 15 | 27 | 34 |