Note to the reader: This is a revised edition of a paper published in Behavioral Neuroscience (1997;111:404–412). The definitive original print version is copyright ©1994 by American Psychological Association.
New figures, text, and links have been incorporated into the revision. Revised HTML (http://nervenet.org/netpapers/Rosen/AudMK97/AudMK.html) copyright ©2000 by Glenn D. Rosen
THE EFFECTS OF SEX AND MK-801 ON AUDITORY PROCESSING DEFICITS ASSOCIATED WITH DEVELOPMENTAL MICROGYRIC LESIONS IN RATS
R. Holly Fitch,* Christine P. Brown,* Paula Tallal,* and Glenn D. Rosen†
*Center for Molecular and Behavioral Neuroscience, Rutgers University, 197 University Ave., Newark, NJ 07102. †Dyslexia Research Laboratory and Charles A. Dana Research Institute, Beth Israel Deaconess Medical Center; Division of Behavioral Neurology , Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston MA 02215; Harvard Medical School, Boston, MA 02115.
Please address correspondence to:
Roslyn Holly Fitch
Biobehavioral Graduate Degree Program
Box U-154
University of Connecticut
Storrs, CT 06268-0154
Email: hfitch@psych.psy.uconn.edu
Neocortical microgyric lesions caused by focal freezing injury lead to defects in rapid auditory processing in adult male rats. Since research suggests that females are less susceptible to the deleterious effects of early neural injury, and that neonatal treatment with neuroprotective agents reduces neural damage from focal ischemic injury, we tested the effects of sex and neuroprotectant exposure on the behavioral consequences of microgyria in rats. Results showed that microgyric males were unable to perform the task at the fastest rate of stimulus presentation, and differed significantly from sham males (who were unimpaired) at this condition. Microgyric females, in contrast, discriminated at all stimulus conditions and did not differ from female shams. Results also showed that microgyric males treated with MK-801 had significantly reduced cortical damage, and were able to perform the discrimination at the fastest condition. Combined results suggest that females are less susceptible to the behavioral effects of neocortical freezing injury, and that MK-801 may ameliorate the behavioral and anatomic consequences of these lesions in male rats.
Freezing injury to the cortical plate of newborn rats leads to the formation of a focal area of cortical microdysgenesis resembling 4-layered microgyria (1–7), a malformation seen in a variety of neurologic disorders including epilepsy, thantophoric dysplasia, and dyslexia (8–15).
We have recently demonstrated that male rats with induced microgyria show auditory processing deficits on a two-tone sequence discrimination task, specifically at total stimulus durations of 332 ms or less (16). These auditory processing deficits are highly similar to those seen in language impaired children, who exhibit performance deficits on a similar two-tone discrimination task at stimulus durations of around 350 ms or less (17–19). It has been suggested that auditory processing deficits seen in both language impaired children and microgyric rats may reflect anomalies in the sensory systems which underlie rapid auditory processing functions critical to phonological perception in humans (18,19). Such a hypothesis is consistent with evidence of structural sub-cortical anomalies in the auditory thalamic nucleus of dyslexic brains (20).
In the current studies we sought to investigate what factors might protect against these detrimental behavioral consequences of early focal neocortical damage. We have shown that neonatal treatment with the neuroprotective agent dizocilpine (MK-801)—a non-competitive N-Methyl-D-aspartate (NMDA) receptor antagonist—significantly reduces the amount of cortical damage seen in adult male rats with focal neonatal freezing injury (21). Although questions about the exact mechanism of action of this agent in this system remain, it appears that MK-801 blocks the cascade of neurotoxic steps that follow hypoxic/ischemic injury. Research has also shown that females exhibit a significant advantage over males in cognitive recovery from brain lesions as measured in premature infants (22), human adults (23), and adult rats (24). These findings are consistent with the observation that males are at greater risk for a wide variety of neurodevelopmental disorders (25), including language-based disorders with phonological processing components (25–29). Evidence of higher male prevalence for language disorder might indicate that males are more susceptible to the effects of early damage to the auditory processing systems which have been shown to be critical to phonological and language development in humans (18,19).
To test the effect of these factors on the behavioral consequences of early neocortical focal damage, we conducted two studies. In the first, male rats received focal bilateral neocortical freezing lesions or sham surgery on P1, with concurrent saline or MK-801 treatment. In the second, male and female rats received either bilateral neocortical freezing lesions on P1 or sham surgery. All subjects were tested in an auditory discrimination paradigm modeled on a task that has shown significant differences between language impaired and control children (but see also 16,17).
Induction of Focal Necrotic Lesions
In Study 1, 6 pregnant female Wistar rats were obtained from Charles-River. On the day after birth (P1), male pups were gathered, randomly assigned to receive either bilateral freezing lesions or sham surgery, and redistributed to mothers in "litters" of 10. Treatment with MK-801 or saline was performed for a whole litter, due to potential interactive effects created by mothers grooming both treated and untreated pups. However, assignment of subjects to lesion and sham groups was balanced within a litter. Focal necrotic lesions were then induced based on a modification of the technique employed by Dvorák and colleagues (1,2), and reported in detail elsewhere (4,6). Briefly, pups were anesthetized via induction of hypothermia, and a small midline incision was made in the skin overlying the skull. For lesion subjects, a cooled (&Mac197; -70°C) 2 mm diameter stainless steel probe was placed for 5 seconds on the skull of lesion subjects, lateral to the midway point between bregma and lambda. The first hemisphere to receive the freezing lesion was randomly assigned. Sham subjects were prepared as above, except that the probe was maintained at room temperature. After placement of the probe the skin was quickly sutured, subjects were uniquely marked with ink injections to the footpads, warmed under a lamp and returned to the mother.
In Study 2, three female Wistar rats were bred in-house by GDR to avoid the potential effects of stress during shipping. At birth, litters were culled to 12 pups, evenly distributed by males and females. Subjects were randomly assigned to receive either bilateral freezing lesions or sham surgery (as above), and again, treatments were balanced within litters. Litters were weaned on P21 and the subjects group-housed (2-3/cage) with like-treated same-sexed littermates until P45, when subjects from each study were individually marked with picric acid and shipped to RHF.
MK-801 Injections
Twenty subjects in Study 1 received an i.p injection of either saline or 2 mg/kg MK-801 (2 mg/ml) 0.5 hours prior to freezing injury, and 6 and 14 hours after surgery. Another 17 animals were given MK-801 doses 0.5 hours prior to freezing injury and 6 hours later. Of the 20 subjects given 3 doses of MK-801, 10 did not survive (6 sham and 4 lesioned), a mortality rate consistent with previously published reports (21). All 17 subjects treated with 2 doses of MK-801 survived. The 6 MK-801-treated bilaterally-lesioned animals were from the 3 X 2 mg/kg group as were 4 of the MK-801-treated unlesioned animals. The remaining 2 MK-801-treated unlesioned animals were randomly selected from the 2 X 2 mg/kg group.
Behavioral Testing
Upon receipt by RHF, subjects were individually housed in tubs. The behavioral testing was performed blind with respect to group. At approximately P70, subjects were put on a water restricted schedule, and received ad libitum access to water for only 15 min. per day.
The behavioral testing paradigm is described in detail in Fitch et al. (16). Subjects were introduced to a modified operant conditioning apparatus for training sessions of 30 to 40 minutes per day. The test apparatus consisted of a plexiglass box, modified by the attachment of a plexiglass tube. The face of the tube was affixed to a plate containing a mechanical switch which the rat could operate with his/her nose, and a drinking tube below the switch. Audio micro-speakers were affixed bilaterally over holes drilled in the plexiglass tube. This apparatus was custom designed to allow shaping of subjects through a series of phases controlled by a Macintosh IIci computer (Apple Computer, Cupertino, CA). Subjects were trained to insert their head into the tube (breaking an emitter-detector beam), and to hold this position for a period of 750 ms before pressing the illuminated nose-button to obtain a water reward. Subjects received white-noise feedback to indicate correct positioning. Once able to consistently perform this task (48 trials/session), subjects were introduced to the auditory discrimination paradigm.
Testing consisted of a go-no go target identification task. Once in position, the subject was exposed to an auditory stimulus which consisted of a two-tone sequence. The subject was required to assess whether this stimulus was a “target” (reinforced), or a non-target sequence (not reinforced). The full presentation of the stimulus was contingent upon proper head-placement of the subject; removal of the head during stimulus presentation resulted in an aborted trial, and a 5 s time-out (all lights extinguished). The same tone sequence was then presented on the next trial. If proper head-position was maintained for the duration of stimulus presentation, then the nose-button was illuminated for a 3 s response interval. A press following the subject’s target resulted in the presentation of water, while a press following a negative sequence resulted in a time-out of 45 s.
The stimuli were generated by a Macintosh IIci computer, and were composed of two ramped sine-wave tones 20 ms in duration, separated by an interstimulus interval (ISI) of 500 ms. The low tone was 1100 hertz and the high tone was 2300 hertz, presented at a supra-threshold intensity of 75 decibels. Only Hi-Lo or Lo-Hi sequences were assigned as targets, and these were counterbalanced across animals and remained constant for each subject across testing sessions. Presentation of target and non-target stimuli in a test session was random with the constraint that half of the presentations be target (to maintain motivation), and that no more than 3 target or non-target sequences occur in succession. Each daily session consisted of forty-eight trials.
After 6 days of testing at the above stimulus parameters (20 ms tone, 500 ms ISI, 20 ms tone), the ISI for all sequences (including targets and non-targets) was reduced to 350 ms. All other parameters, including the assignment of each subject's target, remained constant. At the end of 6 days, the duration of the tones within the stimulus sequence were reduced from 20 to 16 ms each, and the ISI was reduced from 350 to 300 ms. After 6 days of testing the tone durations were reduced to 12 ms and the ISI was reduced to 225 ms for another 6 days of testing. Finally, stimulus parameters were returned to the longest duration (20 ms tone, 500 ms ISI, 20 ms tone), for a final 6 days of testing. Thus there were a total of 30 days of testing, with 6 days at each of 5 conditions defined by incrementally decreased (and finally, increased) stimulus durations.
For each test session the sequence of presentation on each trial, and the corresponding response type (hit, false alarm, correct rejection or miss) and latency to respond, were recorded by a Macintosh IIci computer. All phases of training were controlled by programs written in the software program LabView (National Instruments, Austin, TX) specifically for this purpose.
Histology
After the completion of testing subjects were deeply anesthetized with ketamine and xylazine, and were transcardially perfused with 0.9% saline and 10% formalin. The skulls were extracted from the heads, placed in 10% formalin, and shipped to GDR. There, the brains were removed from the skulls and were placed into fresh 10% formalin for 7 days, before being dehydrated in a series of graded alcohols and embedded in 12% celloidin (c.f., 30). Serial sections were cut coronally at 30 µm and a series of every 10th section was stained for Nissl substance with cresyllecht violet. Using a drawing tube attached to a Zeiss Universal photomicroscope (Carl Zeiss, Inc., New York), both neocortical hemispheres were drawn from the frontal to occipital pole. In addition, the damaged area was traced starting from the first section which showed any architectonic distortion and proceeding until the distortion had unambiguously disappeared. The damaged area was measured from these drawings using NIH Image v1.54 on a Macintosh Centris 650 computer. Total microgyric volume was determined using Cavalieri’s estimation (31, see 16 for further details). The architectonic location of the lesion was also quantified by overlaying the topographic location on a normalized flattened map of the neocortex derived from Zilles (32).
We have previously shown that shorter latencies to respond to target as compared to non-target stimuli provide a sensitive measure of target discrimination (16,33). Research with infants using a similar operant auditory discrimination paradigm has also shown that this latency measure correlates well with percent correct. Therefore, discrimination was assessed within each group as a function of Response type (false alarm/hit) effects on response latency as a function of Condition and Day. Finally, data was compiled into discrimination indices (derived from the difference between false alarm (FA) and hit latencies for each subject, for each day of testing), which were analyzed using multiple factor ANOVAs, with Treatment and Sex as between variables, and Condition and Day as within variables. In many cases one-tailed tests were used to assess replications or a priori hypothesized effects, and in such cases the use of one-tailed tests is noted.
First, however, we assessed whether data from like-treated groups could be pooled. In Study 1, we found no effect of 2 versus 3 injections of MK-801 on treated shams (mean FA/hit difference for 2X MK-801 = 50.6 ms, 3X MK-801 = 43.7 ms), and no effect of MK-801 versus saline treatment on male sham performance (mean FA/hit difference for MK-801 shams = 46 ms, saline treated shams = 34.2 ms), hence these groups were pooled. We also found no significant differences between sham male performance for Studies 1 and 2, and hence all sham males from Studies 1 and 2 were pooled into a single group (n=18). We also found no performance differences for untreated bilaterally lesioned males in Studies 1 and 2, and these groups were also pooled (n=12).
Within-Group Analyses
Response latencies to target and non-target stimuli were analyzed for all groups using multi-factor ANOVAs, with Treatment and/or Study as between variables and Condition, Day, and Response Type (false alarms versus hits) as within variables. Analyses of sham male data showed significant overall discrimination (F1,16=27.6, P<.001), and simple effects showed discrimination at each of the 5 conditions (Cond. 1: F1,16=8.05, P<.01; Cond. 2: F1,16=9.9, P<.005; Cond. 3: F1,16=17.4, P<.001; Cond. 4: F1,16=24.8, P<.0001; Cond. 5: F1,16=14.1, P<.01; all tests one-tail). Analyses of data from bilaterally lesioned males showed overall significant discrimination (F1,10=9.9, P<.02), and significant discrimination at Condition 1 (F1,10=6.02, P<.02, one-tail), Condition 2 ( F1,10=19.3, P<.001, one-tail), and Condition 3 (F1,10=3.96, P<.05, one-tail) but not Condition 4 (F1,10=.24, P=.32, one-tail). There was near-significant discrimination, however, when lesioned males were returned to the slowest condition (F1,10=2.5, P=.073, one-tail).
Analyses on sham and bilaterally lesioned females showed significant discrimination for both groups overall (F1,5=6.05, P<.05; F1,5=15.23; P<.01; one-tail). Simple effects showed near significant or significant discrimination for all 5 conditions for sham females (Cond. 1: F1,5=3.2, P=.07; Cond 2: F1,5=5.4; P<.05; Cond 3, F1,5=10.3, P<.02; Cond 4: F1,5=5.2, P<.05; Cond. 5: F1,5=2.9, P=.08; all tests one-tail) and lesioned females (Cond. 1: F1,5=6.2, P<.05; Cond. 2: F1,5=2.1, P=.1; Cond. 3: F1,5=6.2, P<.05; Cond. 4: F1,5=19.9, P<.01; Cond. 5: F1,5=6.39, P<.05; all tests one-tail).
The MK-801 treated bilaterally lesioned males showed near-significant overall discrimination ( F1,5=2.9, P=.075, one-tail), but failed to show significant discrimination as measured by simple effects at Conditions 1, 2, or 3. Nevertheless, they did show significant discrimination at the fastest condition (Cond. 4: F1,5=4.4, P<.05, one-tail) where untreated bilaterally lesioned males had failed to discriminate. MK-801 treated bilaterally lesioned males also showed significant discrimination when the slowest condition was presented again (Cond. 5: F1,5=7.6, P<.05, one-tail). The causes underlying poor performance by MK-801 treated lesioned males in the initial stages of testing is not clear, especially since MK-801 alone exerted no deleterious effects on sham male performance. Nevertheless, it important to note that these subjects failed to show the rate-specific deficit seen in untreated lesioned males in the current study, and in Fitch et al. (16).
Between-Group Analyses
For males, Treatment (sham versus lesioned) interacted with Condition (F4,112= 2.0, P=.05, one-tail), an effect which derived from significantly better performance for sham as compared to untreated lesioned males at the fastest condition (Cond. 4: F1,28=4.8, P<.02,one-tail; see Figure 1). This effect replicates a previously reported finding (16). Interestingly, there were no differences between groups when returned to the slowest condition (Cond. 5: F1,28=.66, P=.21, one-tail). These results are important, because they reveal that the decrement in lesioned male performance at Condition 4 was not a reflection of decreasing motivation or “failure to learn,” but rather, a processing deficit specific to rapid rates of stimulus presentation.
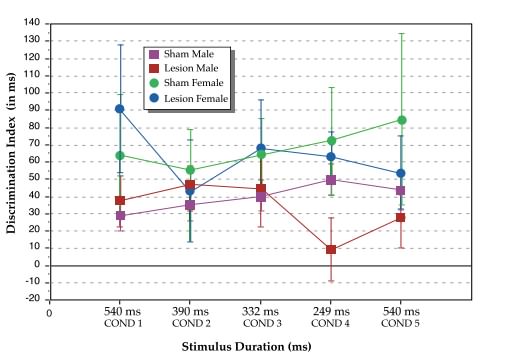 |
| Figure 1. Discrimination indices (vertical axis), as calculated by the false alarm/hit latency difference (in ms), for sham and lesioned male and female rats at the 5 stimulus duration conditions. Total auditory stimulus duration (horizontal axis) given in ms. |
For females, there was no effect of Treatment (sham versus lesioned), overall or at any condition. However, when males and females were analyzed together, a main effect of Sex (F1,38=4.67, P<.05) was observed, with females performing better than males. Analysis of simple effects at each of the 5 conditions revealed a marginal advantage of lesioned females over lesioned males at Condition 1 (F1,38= 3.3, P=.077), and a significant advantage of lesioned females over lesioned males at the fastest condition (Cond. 4: F1,38=4.3, P<.05, see Figure 1). Sham females did not differ significantly from sham males, overall or at any condition.
Analysis of MK-801 treated bilaterally lesioned males, versus untreated lesioned males, revealed an interaction between Treatment and Condition (F4,64=2.2, P<.05, one-tail), which reflected in part a near-significant advantage of MK-801 treated lesioned males over untreated lesioned males at condition 4 (F1,16=2.3, P=.075, one-tail; see Figure 2). There were no significant differences between groups at Conditions 1, 2, 3, or 5. These results, when combined with evidence that MK-801 treated lesioned males but not untreated lesioned males showed significant discrimination at Condition 4, suggest that treatment with MK-801 concurrent to neonatal lesion induction ameliorated rate-specific processing deficits seen at the fastest condition.
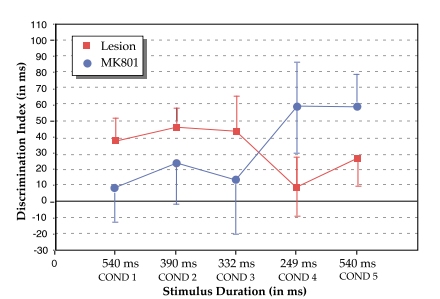 |
| Figure 2. Discrimination indices (vertical axis), as calculated by the false alarm/hit latency difference (in ms), for untreated and MK-801 treated lesioned male rats at the 5 stimulus duration conditions. Total auditory stimulus duration (horizontal axis) given in ms. |
Learning Indices
As a final note, we analyzed the time and amount of reinforcement required for all subjects to learn the initial operant task (pressing the nose button for water), as a function of experimental group. Analyses revealed that sham and lesioned females took significantly longer (F1,38=11.23, P<.002) and more reinforcement (F1,38=6.2, P<.02) to learn the task as compared to sham and lesioned males (MK-801 treated lesioned males were not included in this analysis). We interpret this as a reflection of greater exploratory behavior in females as compared to males during the operant sessions, a phenomenon noted and discussed in a prior study with male and female rats (33). Interestingly, lesioned subjects did not require more time to learn the task, and in fact, as measured by amount of reinforcement, took marginally less (F1,38=3.04, P=.089). MK-801 treated lesioned males took the least time and reinforcement of any group to learn the task.
Histological Analyses
Analyses were performed on the amount of cortical damage for all lesioned subjects as a function of sex, and treatment (MK801 versus untreated males). No significant differences were seen between untreated bilaterally lesioned males and females (F1,15=.09, n.s.), [mean male lesion, total (both hemispheres) with SEM = 8.2 ± 0.94 mm3; mean female lesion = 7.8 ± 1.2 mm3]. Lesioned areas were centered in Par1, HL, and FL with some encroachment into the lateral borders and the caudomedial portions of Fr, and the rostral-most portions of Oc. Two lesions (one male and one female) extended into the Par2/Te border area (see Figure 3). Lesioned males treated with MK-801 showed significantly less damage as compared to untreated lesioned males (F1,15= 12.3, P<.01), [mean MK-801 lesion = 2.9 ± 0.58 mm3], and lesioned areas for this group included predominently Par1, HL, and FL with some extension into the lateral border with Fr (see Figure 3). [Note -- the df reflect the fact that a single MK-801 treated brain was lost to histology due to unexpected death after behavioral testing but before perfusion].
 |
| Figure 3. Topographic location of microgyric damage in untreated lesioned males, untreated lesioned females, and MK-801 treated lesioned males. Areas of damage are depicted on a flattened, normalized map of the neocortex derived from Zilles (32). Each lesion is plotted and areas of overlap are indicated by progressively darker shades of gray. Abbreviations: AI - agranular insular (includes dorsal, posterior, and ventral part); Cg - cingulate cortex (included Cg1-3); FL - forelimb area; Fr - frontal cortex (includes areas Fr11, Fr2, and Fr3 ); Gu - gustatory cortex; HL - hindlimb area; IL - infralimbic area of the medial frontal cortex; MO - medial orbital area; Oc - occipital cortex (includes all subdivisions of Oc1 and Oc2); Par1 and Par2 - primary and secondary somatosensory cortices, respectively; RS - retrosplenial cortex (includes granular and agranular subdivisions); Te- primary auditory cortex, and temporal areas 2 and 3. |
The critical results presented here are summarized as follows.