![]()
http://www.nervenet.org/netpapers/Gerfen/Gerfen1992.html or
HTML edition: html version Copyright © 2000 CR Gerfen.
In: Annual Review of Neuroscience 15:285–320 (1992)
gerfen@helix.nih.gov
Laboratory of Cell Biology
The basal ganglia provide a major neural system through which the cortex effects behavior. Most notable among these effects are those related to the voluntary control of movement, which is compromised by neurodegenerative diseases that involve the basal ganglia. Two such diseases, Parkinson's disease and Huntington's chorea, display a spectrum of movement impairment (Albin et al 1989). Parkinson's disease, which results in the degeneration of dopaminergic systems inthe basal ganglia, produces a disability to initiate desired movements. One the other hand, Huntington's chorea, which results in the degeneration of the major projection neurons of the basal ganglia, is characterized by uncontrolled movements. The complexity of these and other disorders that accompany basal ganglia dysfunction suggest its broad role in the most subtlest components of voluntary movement. That memory, motivational, and emotional aspects of movement behavior are affected by this neural system is related to the fact that the striatum, which is the principal component of the basal ganglia, receives inputs from virtually all cortical areas (Carman et al 1965, Kemp and Powell 1970, Webster 1961) including limbic-related areas (Heimer and Wilson 1975).
How the striatum processes cortical inputs is central to the function of the basal ganglia.
The organization of corticostriatal systems has been described in terms of parallel processing mechanisms (Alexander et al 1986). According to this scheme, segregated parallel circuits connect limbic, prefrontal, oculomotor, and motor cortical areas through subregions of the basal ganglia with ventral tier thalamic nuclei that feed back to those same cortical areas. These functional circuits provide a conceptual framework for relating subregions of the basal ganglia to specific aspects of behavior, much as cytoarchitecturally defined cortical areas are defined. Disinhibition has been proposed as the basic mechanism by which these basal ganglia circuits affect behavior (Chevalier et al 1985, Deniau and Chevalier 1985, Hikosaka and Wurtz 1983b). Basal ganglia output nuclei, the entopeduncular nucleus (internal globus pallidus in primates) and the substantia nigra pars reticulata, provide tonic inhibition of ventral tier thalamic nuclei and the superior colliculus. Disinhibition of these inhibitory pathways results from cortical excitation of inhibitory-striatal projections to the entopeduncular nucleus and substantia nigra (Chevalier et al 1985, Deniau and Chevalier 1985). Thus, movements initiated in response to sensory, memory, or motivationally contingent cues have been correlated with pauses in the tonic activity of substantia nigra output neurons (Hikosaka and Wurtz 1983ab). The opposed tonic activity of these neurons is regulated, in part, by the striatal outputs to the globus pallidus (external segment of the globus pallidus in primates). Increased activity in this pathway results, by way of polysynaptic connections through the globus pallidus and subthalamic nucleus (Kita et al 1983, Kita and Kitai 1987ab), in increased activity of entopeduncular (internal globus pallidus inprimates) and substantia nigra neurons. The important contribution of increased tonic activity of these neurons through this indirect pathway to the execution of movements has been suggested (Alexander and Crutcher 1990). Balanced opposition of cortically driven striatal output systems thus appears to be responsible for the generation of normal movements.
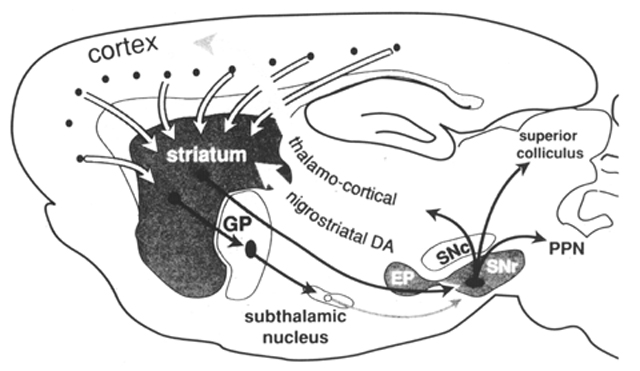
The neuroanatomical substrates that underlie the processing of cortical inputs to produce this balanced opposition reflect organizational schemes that are common to most regions of the striatum. Unlike the cortex, the striatum lacks the clear cytoarchitectural definition that has aided analysis of information processing through layer-specific cortical connections (Jones 1984). Although the striatum lacks cytoarchitectural definition, subpopulations of striatal neurons are organized in functional compartments. This compartmental organization can be shown in some cases to be comparable to cytoarchitectural features of the cortex, and in other cases to represent an organization unique to the striatum. Two such levels of compartmental organization are described that are not exclusive but represent overlapping sets of subpopulations of striatal output neurons. The first level of compartmental organization is defined by the segregation of striatal output neurons into patch and matrix compartments (Bolam et al 1988, Gerfen 1985, Kawaguchi et al 1989), which are related to laminarand regional aspects of cortical organization (Donoghue and Herkenham 1986, Gerfen 1984, 1989). The second level of compartmental organization is the separation of projections to the external segment of the globus pallidus and to the substantia nigra (Albin et al 1989, Gerfen et al 1990, Kawaguchi et al 1990). The major output neurons of the basal ganglia are the GABAergic neurons of the substantia nigra pars reticulata and the entopeduncular nucleus (internal segment of the globus pallidus in primates). For this review these two cell groups are considered part of the same extended nucleus and striatonigral projections refer to inputs to this extended nucleus. Neurons giving rise to striatopallidal and striatonigral projections are separate and intermingled in both striatal patch and matrix compartments. The organization of striatopallidal and striatonigral systems provides for the conversion of the excitatory inputs to the striatum into balanced opposed modulation of output neurons of the basal ganglia (Albin et al 1989, Gerfen et al 1990). The cellular and molecular mechanisms responsible for regulating the balance between these systems establish the capacity for the basal ganglia to affect the selection of specific behaviors. The principal component of the basal ganglia is the striatum, which comprises the caudate, putamen, and accumbens nuclei. One neuron cell type, the medium spiny neuron, accounts for 90–95% of the striatal neuron population. These neurons have a medium-sized cell body, approximately 20–25 µm in diameter, from which radiate branched dendrites that are densely laden with spines (Kemp and Powell 1971, Wilson and Groves 1980). The dendritic arbors extend in a domain approximately 150–250 µm in diameter such that neighboring neurons share common inputs. As they are the predominant neuron cell type in the striatum, medium spiny neurons are the major target of extrinsic afferents. Cortical and thalamic inputs provide excitatory inputs that make asymmetric synaptic contact mainly with the heads of the spines (Bouyer et al 1984, Hattori et al 1978, Somogyi et al 1981). Dopamine fibers from the midbrain cell groups are the other major source of extrinsic input to these neurons, which make symmetric synaptic contact primarily with the necks of dendritic spines and on the interspine dendritic shafts (Bouyer et al 1984, Freund et al 1984). Other inputs to medium spiny neurons come primarily from two major sources within the striatum, from the local axon collaterals of other medium spiny neurons or from striatal interneurons (Wilson and Groves 1980). Intrinsic neurons, whose axons do not exit the striatum, comprise about 10% of the striatal neuronal population and have a profound role in striatalorganization. Among the neurons that have been clearly identified in this class are the large aspiny neurons that utilize acetylcholine as a transmitter (Bolam et al 1984), and several types of medium aspiny neurons, which include those that contain somatostatin (DiFiglia and Aronin 1982) and neuropeptide Y (Vincent and Johansson 1983) and those that contain the calcium-binding protein parvalbumin (Cowan et al 1990, Gerfen et al 1985). Figure 1. Schematic representation of the major connections of the basal ganglia. The main component of the basal ganglia, the striatum, receives inputs from the cortex. Two major striatal output pathways target the globus pallidus (GP) and entopeduncular (EP) substantia nigra pars reticulata (SNr) complex. Dopamine (DA) neurons in the substantia nigra pars compacta (SNc) receive inputs from the striatum (not diagrammed) and provide feedback via the nigrostriatal DA pathway. EP and SNr neurons provide inhibitory inputs to the pedunculopontine nucleus (PPN), superior colliculus, and thalamus. Nigrothalamic inputs target intralaminar nuclei that provide feedback to the striatum (not shown) and ventral tier thalamic nuclei that provide inputs to the frontal cortex. The basic organizational scheme of the basal ganglia is diagramed in Figure 1. Pyramidal cortical neurons located primarily in layer 5, but also some in layers 2 and 3 and 6, provide inputs to striatum (Ferino et al 1987, Jones et al 1977, Royce 1982, Wilson 1987). These inputs utilize the amino acid glutamate as a neurotransmitter (Spencer 1976), and thus provide excitatory inputs to the striatal medium spiny neurons (Kitai et al 1976). Neurons in the intralaminar thalamic nuclei and the parafascicular nucleus (Gerfen et al 1982, Herkenham and Pert 1981, Krettek and Price 1977) also provide excitatory inputs to striatal medium spiny neurons (Kitai et al 1976). Striatal medium spiny neurons are the primary source of striatal projections (Grofova 1975). These neurons are normally relatively quiescent, and activity in the output pathways is generated as a result of cortical and thalamic excitatory inputs (Kitai et at 1976, Wilson 1981). Another major source of inputs to the striatum is from the midbrain dopaminergic cell groups in the ventral tegmental area, substantia nigra, and retrorubral area (Freund et at 1984, Gerfen et at 1987, Jimenez-Castellanos and Graybiel 1987). As is discussed in some detail below, this dopaminergic input appears to modulate the responsiveness of striatal output neurons to cortical and thalamic inputs. Medium spiny striatal neurons utilize gamma amino butyric acid (GABA) as their principal transmitter (Kita and Kitai 1988) and thus provide inhibition to the targets of striatal output in the globus pallidus, entopeduncular nucleus (the internal segment of the globus pallidus in primates), and the substantia nigra (Chevalier et at 1985, Deniau and Chevalier 1985, Kita and Kitai 1988). The principal neuron type in the globus pallidus is GABAergic and provides inhibitory inputs to the subthalamic nucleus and to the substantia nigra (Smith et at 1990). Neurons in the subthalamic nucleus provide an excitatory input to the substantia nigra (Kita and Kitai 1987ab, Nakanishi et at 1988). This latter input to the substantia nigra represents an indirect pathway, which is responsible, in part, for the tonic activity of GABAergic neurons in the substantia nigra pars reticulata. The direct striatonigral pathway provides principally inhibitory inputs to both the dopaminergic and GABAergic neurons in the substantia nigra (Chevalier et at 1985, Deniau and Chevalier 1985). As mentioned, nigral dopaminergic neurons are the source of the feedback pathway to the striatum. Nigral GABAergic neurons, located in the substantia nigra pars reticulata and in the entopeduncular nucleus, provide inhibitory inputs to the intermediate layers of the superior colliculus, the pedunculopontine nucleus, and the thalamus (Gerfen et at 1982).
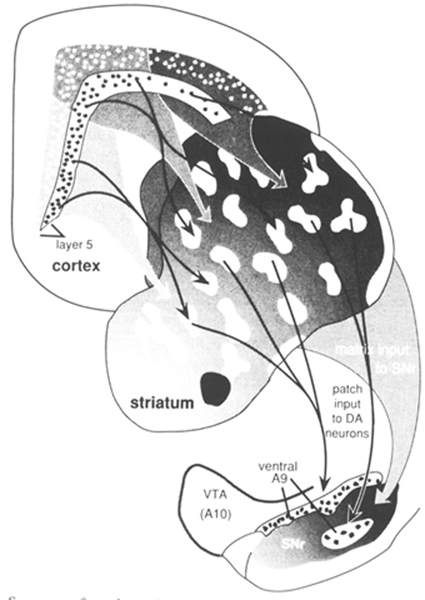
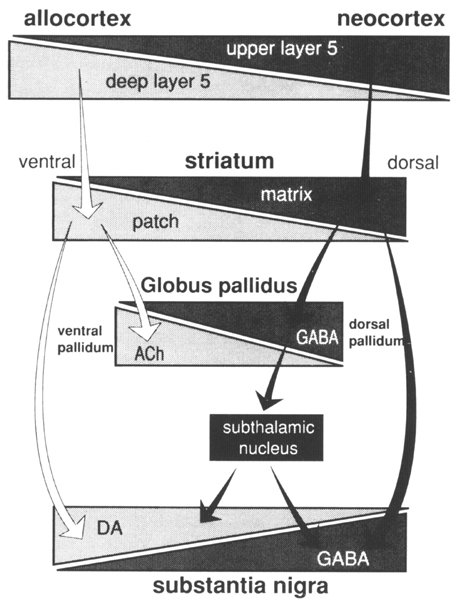
Many connections have been omitted from the general scheme described so as to focus on the basic elements detailed in this review. Two levels of compartmental organization that may be overlain on the general scheme of basal ganglia connections are described- The first is the patch-matrix compartmental organization of the striatum and the second is the organization of separate striatopallidal and striatonigral systems. The striatal patch and matrix compartments are defined on the basis of specific neurochemical markers and the connections of the underlying striatal neurons. The striatal patch compartment is defined by areas of dense alpha-opiate receptor binding (Herkenham and Pert 1981) and areas of low acetylcholinesterase labeling, also referred to as striosomes (Graybiel and Ragsdale 1978). The striatal matrix compartment, which is complementary to the patches, is composed of neurons that contain a 28 kD calcium-binding protein (calbindin) and a rich plexus of somatostatin immunoreactive fibers (Gerfen 1985, Gerfen et al 1985). These neurochemical markers display a consistent complementary pattern in the rat throughout the majority of both the dorsal and ventral striatum, although within the medial-aspect of the nucleus accumbens these patterns are not as distinct (Voorn et al 1989). Thus, these markers may be used to define the mosaic organization of the striatum into distinct patch and matrix compartments. These compartments have been well characterized in the rat, and a similar organization obtains in the primate and cat. Other neurochemical markers, most notably the immunohistochemical distribution of the peptides enkephalin and substance P (Beckstead and Kersey 1985, Gerfen 1984, Graybiel et al 1981), and some of the input (Malach and Graybiel 1986) and output (Gimenez-Amaya and Graybiel 1990) connections of the striatum, display patterns of heterogeneity in the striatum that sometimes are and sometimes are not consistent with the patch and matrix compartments as defined here. As is discussed below, these patterns reveal multiple levels of compartmental organization, only some of which are related to the patch-matrix compartments. Although the neurochemical markers described above have been useful in defining the patch-matrix compartments, this organization appears to be related to the segregation of separate populations of striatal medium spiny neurons that have distinct input-output connections (Gerfen 1984, 1985, Gerfen et al 1985, Kawaguchi et al 1989). In rats, retrograde axonal tracing studies show that both patch and matrix neurons project to the substantia nigra, but that patch neurons provide inputs to the location of dopaminergic cells, most specifically to the ventral tier of dopaminergic neurons in the pars compacta and dopaminergic cell islands in the pars reticulata, whereas matrix neurons provide inputs to the location of the GABAergic neurons in the substantia nigra pars reticulata (Gerfen 1984, 1985, Gerfen et al 1985). Calbindin immunoreactivity, which labels striatonigral projection neurons in the matrix, confirms by its specific distribution in terminals in the non-dopaminergic parts of the substantia nigra pars reticulata, the patch-matrix organization of the striatonigral pathway (Gerfen et at 1985). A similar pattern of calbindin immunoreactivity in the striatonigral pathway of primates (Gerfen et al 1985) and a later study in the cat (Jimenez-Castellanos and Graybiel 1989) suggests that a similar dichotomy of patch and matrix striatonigral pathways occurs in these species as well. Multiple studies have demonstrated that the dendrites of patch and matrix medium spiny neurons remain restricted, for the most part, to the compartment of the parent neuron (Bolam et al 1988, Gerfen 1985, Herkenham et al 1984, Kawaguchi et at 1989). This is an important characteristic, as it suggests that inputs that are confined to the patch compartment will affect only patch output neurons, whereas inputs directed to the matrix will affect matrix output neurons. Dopaminergic neurons in the midbrain ventral tegmental area, substantia nigra, and retrorubral area provide a massive input to the striatum that is compartmentally organized (Gerfen et at 1987a, Herkenham et at 1984a, Jimenez-Castellanos and Graybiel 1987). Matrix-directed dopaminergic projections arise from a continuous group of neurons that are located in the ventral tegmental area, the dorsal tier of the substantia nigra pars compacta, and the retrorubral area. Patch-directed dopaminergic neurons arise from ventral substantia nigra pars compacta neurons and from dopaminergic cells clustered in islands in the pars reticulata. Moreover, the matrix-directed dopaminergic neurons also express calbindin immunoreactivity, whereas dopaminergic neurons projecting to the patches do not (Gerfen et at 1987b). These studies establish the existence of separate dorsal and ventral tier dopaminergic mesostriatal systems that are directed to the striatal patch and matrix compartments and that are also biochemically distinct. An additional feature of this organization is that the dorsal tier dopaminergic neurons have dendrites that remain confined to the area of the dopaminergic neurons, whereas the ventral tier, patch-directed neurons have dendrites that extend into the parts of the substantia nigra pars reticulata where GABAergic neurons are located (Gerfen et al 1987). This is of possible interest, given the reports that the latter dopaminergic dendrites have been reported to release dopamine (Cheramy et at 1981). As described above, the output of the striatal patch compartment specifically targets this ventral set of dopaminergic neurons. Whether the significance of this input is related to the dendritic release of dopamine by these neurons remains to be determined. It is of interest that the dorsal tier dopaminergic neurons that provide the major input to the striatal matrix receive little in the way of inputs from the striatum, but rather receive inputs from other areas, such as the amygdala (Gonzales and Chesselet 1990). Several studies have demonstrated that corticostriatal inputs are heterogeneously distributed within the striatum (Donoghue and Herkenham 1986, Gerfen and Sawchenko 1984, Goldman-Rakic 1982, Ragsdale and Graybiel 1981). A recent study of the compartmental organization of the corticostriatal projection in the rat suggests that the patch-matrix organization of the striatum is related to the laminar organization of the cortex (Gerfen 1989). The majority of corticostriatal neurons are located in layer 5 and in the deeper parts of layer 3. By using the anterograde axonal tracer PHAL (Gerfen and Sawchenko 1984), it was possible to examine the projections of sublaminae of layer 5 to the striatum. Results demonstrated that corticostriatal neurons in the deep parts of layer 5 project preferentially to the striatal patch compartment, whereas projections from upper layer 5 and from supragranular layers project primarily to the striatal matrix compartment. Although all cortical areas examined project to both striatal compartments, the relative input to each compartment varies such that periallocortical areas provide a dense input to the patch compartment, whereas the input from neocortical areas to the patch compartment is relatively sparse. Retrograde tracing studies suggest that the trends in the relative contribution of corticostriatal inputs to the two compartments is related to the numbers of deep versus superficial layer 5 corticostriatal neurons in periallo- and neocortical areas; neocortical areas have relatively few deeper layer 5 neurons that contribute inputs to the striatum (Ferino et al 1987, Wilson 1987). The relative paucity of inputs from neocortical areas to the patch compartment may explain reports from tracing studies in primates in which injections into neocortical areas appear to label inputs primarily to the matrix compartment (Goldman-Rakic 1982). In this regard, it may be significant that the area of the primate putamen that receives inputs from the highly evolved neocortical areas, the primary motor and sensory cortices, appears to have a very diminished patch compartment. Thus, in primates, in which neocortical areas are greatly expanded as compared with rodents, the relative contribution of cortical inputs to the striatal patch compartment may be diminished. Nonetheless, the laminar organization of corticostriatal inputs would appear to be a fundamental determinant of the functional significance of the striatal patch and matrix compartments (Figure 2). Figure 2. Summary of patch-matrix compartmental organization of corticostriatal and striatonigral pathways. Corticostriatal neurons in the deep parts of layer 5 provide inputs to the striatal patch compartment, whereas superficial layer 5 neurons provide inputs to the striatal matrix. Patch neurons provide inputs to the location of dopaminergic neurons in the ventral tier of the substantia nigra pars compacta and islands of dopamine neurons in the pars reticulata. Striatal matrix neurons provide inputs to the location of GABAergic neurons in the substantia nigra pars reticulata (SNr). Relating the patch-matrix organization of the striatum to the laminar organization of the cortex merely begs the question as to the functional significance of cortical output organization. The laminar organization of the cerebral cortex is related to the aggregation of pyramidal neurons that have common axonal projection targets (Gilbert and Kelly 1975, Jones 1984). Thus, layer 6 cortical output neurons provide inputs to the thalamus, layer 5 neurons provide inputs to other subcortical structures, including the striatum, brainstem, and spinal cord, and supragranular layer neurons are the major source of corticocortical projections. In this regard a number of different subtypes of corticostriatal neurons have been described that differ on the basis of axon collaterals to other brain sites, such as to the cerebellum, pyramidal tract, thalamus, contralateral cortex, and contralateral striatum (Donoghue and Kitai 1981, Jinnai and Matsuda 1979, Royce and Bromley 1984, Wilson 1987). Corticostriatal neurons also show differences in local axon collaterals with different patterns of laminar and regional spread (Wilson 1987). Although the laminar and sublaminar distributions of subtypes of corticostriatal neurons have not been established, these types of connectional distinctions presumably underlie the functional significance of the laminar origins of corticostriatal projections to the striatal patch and matrix compartments. There are other aspects of cortical organization, such as the radial columnar organization, that may contribute to heterogeneously distributed striatal afferents. Such organization is not to be confused, however, with the laminar relationship to the striatal patch-matrix organization. Previous studies have suggested a relationship between limbic-related areas, such as the amygdala (Ragsdale and Graybiel 1988) and prelimbic cortex (Donoghue and Herkenham 1986, Gerfen 1984) and the striatal patches, and between sensorimotor cortical areas and the striatal matrix (Donoghue and Herkenham 1986, Gerfen 1984). In the rat, however, it appears that both prelimbic and sensorimotor cortical areas provide inputs to both compartments (Gerfen 1989). Nonetheless, the relative contribution of inputs to the striatal compartments from limbic, or periallocortical, and neocortical areas varies markedly, such that the limbic association with the patch compartment and the neocortical association with the matrix compartment may have some functional utility. On the one hand, such functions may be considered in terms of the regional connections of the striatum. Allo- and periallocortical areas project principally to the ventral striatum, and neocortical areas project principally to the dorsal striatum (Heimer and Wilson 1975). It is on the basis of this regional organization that the dichotomy in limbic- and nonlimbic-related striatal regions have been considered (Haber et al 1985, Heimer and Wilson 1975). Yet such generalizations raise the difficulties of defining limbic and nonlimbic functions. Although the concept of the limbic system has evolved, the original neuroanatomical, functional, and phylogenetic dichotomy of medial and periallocortical areas subserving emotional and visceral functions and lateral, neocortical areas subserving cognitive and sensorimotor functions is generally still accepted (Swanson 1987). As Swanson points out, however, recent appreciation of neuroanatomical interrelationships have blurred the simple dichotomy of such functions. As both allo- and periallocortical and neocortical areas provide inputs to the striatum, in this structure ascribing a functional role to particular inputs is most difficult. Another approach is suggested. Rather than attempt to define the type of information conveyed to the striatum in behavioral terms, it is suggested that regional transitions in both cortical and basal ganglia organization be examined. Although discussion of the issues of cortical evolution is beyond the scope of this review, it is probably noncontroversial to suggest that a major transition from allo- to neocortical areas is characterized by increased specialization of function typified by increased laminar organization. This perhaps has been best discussed in the context of the evolution of thalamocortical connections, i.e. nonspecific projection systems have been supplanted through evolution with more specific connections (Herkenham 1986). In the context of corticostriatal systems, as discussed above, there is a transition from periallocortical to neocortical areas of deep layer 5 projections to the patch compartment to superficial layer 5 projections to the matrix compartment (Gerfen 1989). In the striatum, this transition of corticostriatal patch-matrix systems is exemplified by the relative paucity of the patch compartment in striatal areas receiving neocortical inputs, particularly in the putamen of the primate. There are other indices of regional transition in the striatum. For example, two neurochemical markers of the matrix compartment, somatostatin and calbindin immunoreactivity, are highest in the ventral striatum and diminish dorsally [please check polarity of argument with Chip Gerfen] (Gerfen et al 1985). Other peptides, such as substance P and dynorphin, also show dorsal-ventral regional variations, which are discussed below. A regional transition occurs in the composition of the neurons of the globus pallidus: the dorsal pallidum is composed of mainly GABAergic neurons with infrequent cholinergic neurons, whereas in the ventral pallidum this mixture of neurons is reversed (Grove et al 1986). Peptides expressed in striatopallidal afferents show regional variations: enkephalin is densest in terminals in the dorsal pallidum, whereas substance P is densest in terminals in the ventral pallidum (Alheid and Heimer 1988, Haber and Nauta 1983). Finally, in the substantia nigra, dopaminergic neurons are concentrated in the dorsal pars compacta and ventral tegmental area, whereas GABAergic neurons are distributed in the ventrally located pars reticulata. These regional variations in basal ganglia organization are shown in a diagram, including the patch-matrix compartmental connections, in Figure 3. One of the connections in this diagram is hypothesized. Although it is known that the striatum provides inputs to cholinergic neurons in the pallidum (Grove et al 1986), it is not known whether these arise from patch compartment neurons.
Figure 3. Diagrammatic representation of regionaltransitions in the components of patchmatrix compartmentalconnections. See text for details. (ACh, acetylcholine; DA, dopamine; ENK, enkephalin; SP, Substance P.)
The regional variations in basal ganglia organization are suggested to be an extension of the transition of periallocortical to neocortical subcortical circuits. Alheid and Heimer (1988) have proposed that the subcortical connections of allo- and neocortical areas share common general organizational schemes whose specific elements reflect a transitionin the final targets of these systems and in the feedback mechanisms they employ. The regional transitions in the basal ganglia may be considered in this context. Some outputs of the ventral striatum target cholinergic (Grove et al 1986) and dopaminergic neurons (Nauta et at 1978), two neurochemical systems that provide direct feedbacks to the cortex and striatum. Although there appears to be some cholinergic input to the reticular nucleus of the thalamus from the basal forebrain cell groups (Hallanger et at 1987), the majority of cholinergic neurons in the ventral and dorsal pallidum appear to provide principally ascending cortical projections (Grove 1988 , McKinney et al 1983, Saper 1984). Conversely, the main outputs of the dorsal striatum target GABAergic neurons in the globus pallidus and substantia nigra and, through the thalamic connections of the latter, provide a more indirect feedback to the cortex and striatum (Wilson 1990). It is suggested that these two types of circuits are contained in the connections of the patch and matrix compartments, respectively (Gerfen 1984, 1985). In many regions of the striatum, both of these circuits exist. Rather than specify that the patch compartment conveys limbic information in dorsolateral patches, it is suggested that the type of neuroanatomical circuitry that is typical of the "limbic-ventral" striatum is retained in the dorsal striatum in the connections of the patch compartment. This is analogous to the emergence of neocortical organization in which the type of connections of older cortical areas is retained to some extent but supplanted by new features of organization (Herkenham1986). Thus striatal patch-matrix compartments may be viewed as two phylogenetically distinct neuroanatomical circuits through which cortical information is processed. Regionally, the mix of these two circuit systems varies such that in the ventromedial striatum, allo-and periallocortical circuitry dominates, whereas in the dorsolateral striatum, neocortical circuitry dominates. In much of the striatum the two circuits coexist, and interactions between them may provide mechanisms for regulating the balance in the striatopallidal andstriatonigral systems. As described above, striatal medium spiny neurons are segregated into separate populations that form the basis of the patch and matrix compartments, whose connections are related to the laminar (Gerfen 1989) and regional (Donoghue and Herkenham 1986, Gerfen 1984) organization of the cortex. Medium spiny neurons may also be categorized on the basis of their projections to the globus pallidus, entopeduncular nucleus, and substantia nigra. In a recent study (Kawaguchi et al 1990), medium spiny neurons were intracellularly injected with biocytin, and both the local axon collateral andprojection axons of these neurons were traced. Many neurons extend axon collaterals to each of the target nuclei of striatal outputs; however, the relative extent of the arborization in a particular target area provides the basis for defining two basic striatal output neurons. One type, the striatopallidal neurons, extends an axon that forms a dense arborization within the globus pallidus. These neurons do not appear to extend an axon collateral past the globus pallidus. A second type, the striatonigral neurons (including those with projections to the entopeduncular nucleus), extends an axon collateral in the globus pallidus that does not ramify in this nucleus, and axon collaterals that extend to and arborize extensively in the entopeduncular nucleus and/or substantia nigra. The local axon collateral of most striatopallidal and striatonigral neurons extends in a domain approximately equal to their dendritic domain, which is on average 250–350 microns in diameter. A subtype of striatopallidal neuron, however, extends a local axon collateral over a distance of more than 1 mm. Although these studies examined a relatively limited number of striatal neurons, striatopallidal neurons with extended local axon collaterals appear to be infrequent. Studies employing injections of retrogradely transported markers into the globus pallidus and substantia nigra have provided estimates of the relative numbers of striatopallidal and striatonigral neurons (Loopuijt and Kooy 1985). These numbers must be considered estimates, given the findings that striatonigral neurons extend an axon collateral into the globus pallidus, albeit a small one (Kawaguchi et al 1990). Since determining the extent of uptake of any retrogradely transported marker by either fibers of passage or small collaterals is impossible, these techniques cannot provide exact numbers of the striatal output types. Nonetheless, there is some consensus that the numbers of striatopallidal neurons and striatonigral neurons are approximately equal. Moreover, both types of neurons appear to be somewhat intermingled (Gimenez-Amaya and Graybiel 1990) and to be present in roughly equal numbers in both striatal patch and matrix compartments (Gerfen and Young 1988). As discussed above, striatonigral neurons in the patch and matrix compartments project differentially to the location of dopaminergic and GABAergic neurons in the substantia nigra (Gerfen 1985). The question is raised as to whether there is a similar difference in the projection targets of striatopallidal patch and matrix neurons. Most, and perhaps all, striatal output neurons express glutamic acid decarboxylase (GAD) immunoreactivity (Kita and Kitai 1988) and thus presumably utilize GABA as a neurotransmitter. In addition, striatopallidal and striatonigral neurons contain different sets of neuropeptides. The first suggestion of the biochemical distinction between these output neurons was provided by immunohistochemical studies. Enkephalin immunoreactivity is densely distributed interminals in the globus pallidus but only sparsely in the substantia nigra, whereas substance P immunoreactivity is sparse in terminals in the dorsal globus pallidus, though dense in terminals in the ventral pallidum and in the substantia nigra (Alheid and Heimer 1988, Haber and Nauta 1983). Similarly, dynorphin immunoreactivity is also dense in the substantia nigra but sparse in the globus pallidus (Vincent et al 1982). Thus, immunohistochemical studies suggest that striatopallidal neurons express enkephalin, whereas striatonigral neurons express substance P and dynorphin. That the differential expression of peptides by these output neurons is not absolute is suggested by the finding that a certain percentage of striatal neurons co-express substance P and enkephalin immunoreactivity (Penny et al 1986). The patterns of peptide immunoreactivity in the striatum have led to some confusion regarding the distribution of neurons expressing a given peptide relative to the patch-matrix compartmental organization of the striatum. A number of early studies described the patterns of both enkephalin and substance P immunoreactivity as displaying heterogeneous patterns that correlated, to some extent, with the patch-matrix compartments of the striatum (Beckstead and Kersey 1985, Gerfen 1984, Graybiel et al 1981). These studies revealed little in the way of distinct cellular labeling, and the compartmental patterns were primarily of immunoreactivity of indistinct neuropil labeling. Morerecent studies have shown peptidergic immunoreactive neurons to be more evenly distributed in both compartments (Penny et al 1986). Most likely these different patterns of immunoreactive labeling are related to the parameters of fixation (Graybiel and Chesselet 1984). Nonetheless, the patterns of immunoreactive labeling suggest that the relative expression of different peptides differs regionally in the patch and matrix compartments. As the determinants of immunoreactive labeling of striatal cells remain unclear, in situ hybridization histochemical procedures have been used to characterize the biochemical phenotype of striatal neurons (Gerfen and Young 1988). Results show that the majority of striatopallidal neurons express enkephalin mRNA, whereas the majority of striatonigral neurons express both dynorphin and substance P mRNA. Additionally, approximately 50–60% of both striatal patch and matrix neurons express each peptide mRNA, however, the relative expression of dynorphin mRNA is higher per cell in patch than in matrix neurons in the dorsal striatum, whereas in the ventral striatum there is a more equal relative expression in both compartments. Conversely, substance P mRNA shows an opposite relative expression pattern, as it is higher in ventral striatal patch neurons than in matrix neurons and is roughly equally expressed in both compartments in the dorsal striatum. The patterns of peptide mRNA in situ hybridization histochemistry labeling are consistent with immunoreactive patterns of labeling. Striatonigral and striatopallidal neurons may thus be generally characterized by their respective expression of substanceP/dynorphin and enkephalin. Although these expression patterns characterize the majority of striatal output neurons, some neurons express combinations of peptides that do not strictly adhere to this simplified scheme. Estimates of between 10 and 25% of the striatal neuron population have been suggested for such neurons. One subtype of striatal neuron that projects principally to the globus pallidus and expresses the mRNA encoding the tachykinin neurokinin B has been shown to also contain both enkephalin and substance P (Burgunder and Young 1989). This neuron type represents approximately 15% ofthe striatal population and accounts for some of the neurons that co-express enkephalin and substance P. The rapid advances that have been made in the past few years in cloning G-protein-coupled receptors, which include dopamine receptors, has provided the ability to further characterize striatal neurons with in situ hybridization histochemistry (ISHH) (Figure 4). The D1 and D2 dopamine receptors, which are characterized by their respective stimulation and inhibition of adenylyl cyclase activity (Kebabian and Calne 1979, Stoof and Kebabian 1981), have been recently cloned (Bunzow et al 1988, Dearry et al 1990, Monsma et al 1990 , Sunahara et al 1990, Zhou et at 1990). In situ hybridization histochemical localization of mRNA encoding these receptors has shown that, for the most part, the D1 dopamine receptor is expressed by striatonigral neurons that also contain substance P and dynorphin mRNAs (Gerfen et al 1990), whereas the D2 dopamine receptor is expressed by striatopallidal neurons that also contain enkephalin mRNA (Gerfen et al 1990, Le Moine et al 1990). The somewhat specific expression of the D1 and D2 dopamine receptors by the two striatal output pathways is consistent with receptor binding studies that have shown D1 receptor binding in the substantia nigra and D2 receptor binding in the globus pallidus that originates from striatal output pathways (Beckstead 1988, Harrison et al 1990, Richfield et at 1989). Thus, both in situ hybridization histochemical studies and receptor-binding studies suggest the respective expression of D1 and D2 dopamine receptors in striatonigral and striatopallidal neurons. Figure 4. Striatal neurons retrogradely labeled with the fluorescent dye fluorogold after injection into the substantia nigra combined with darkfield illumination of silver grains produced by ISHH labeling with 35S-labeled oligonucleotide probes for (A) substance P (SP), (B) the D, dopamine receptor (D1), (C) enkephalin (ENK), and (D) the D2 dopamine receptor (D2). Striatonigral neurons show ISHH labeling for both substance P (A, solid arrows) and the D, doparninereceptor (B, solid arrows). Striatal neurons that are unlabeled by fluorogold, and presumably project to the globus pallidus, show ISHH labeling for both enkephalin (C, open arrows) and the D2 dopaminereceptor (D, open arrows). These studies do not suggest an exclusive distribution in these neurons, however. A subset of striatal output neurons appears to express both the D1 and D2 dopamine receptors. The 15–20% of striatal neurons that express the peptide neurokinin B appear to express both receptors. Thus, neurokinin B provides a marker of neurons that co-express the D1 and D2 dopamine receptors. Although mRNAs encoding both D1 and D2 dopamine receptors have been cloned and used to characterizestriatal neuronal populations, there exists the possibility that further subtypes of these receptors may be identified in the future. Already, two subtypes of the D2 receptor have been cloned, one of which differs by the insertion of an extra piece in the third cytoplasmic domain (Monsma et al 1989), and another that differs sufficiently from the D2 receptor to be designated as the D3 receptor (Sokoloff et at 1990). This latter receptor is localized principally in the ventral striatum and nucleus accumbens and shares many of the pharmacologic properties of the D2 receptor. Additionally, evidence suggests that a D1 receptor is coupled to the phosphoinositide second messenger pathway (Felder et al 1989, Mahanet al 1990, Undie and Friedman 1990) and so is probably distinct from that which has already been cloned. Fitting these other subtypes of dopamine receptors into the currently described characterization of striatonigral and striatopallidal neurons will obviously have to await their identification. The recognition that Parkinson's disease results from the degeneration of the nigrostriatal dopaminergic system is one of the cornerstones of the neurochemical basis of behavior (Hornykiewicz 1966). In the 1970s, studies demonstrated that dopamine regulates the expression of peptide levels in the striatum with the finding that chronic neuroleptic treatments result in an elevation of striatal enkephalin levels (Hong et al 1978). These studies initiated a line of research that has provided a strategy for examining the role of dopamine in modulating striatal output neurons. Following the work of Hong and colleagues (1978), numerous studies have shown that a decrease in dopamine action in the striatum, with either neuroleptic treatments or with 6-hydroxydopamine (6-OHDA)-induced dopamine striatal deafferentation, results in an increase in enkephalin peptide and mRNA levels in the striatum but a decrease insubstance P immunoreactivity and mRNA levels (Bannon et al 1986, Gerfen et al 1991, Hanson et al 1981a, Hong et al 1983, Mocchetti et al 1985, Normand et al 1988, Sivam et al 1986, Tang et al 1983, Young et al 1986). Pharmacologic treatments that increase dopaminergic action with either amphetamine or dopamine agonists result in an elevation of striatal levels of both substance P- and dynorphin-immunoreactivity and mRNA (Gerfen et al 1991 , Hanson etal 1981b, Li et al 1987, 1988). Taken together with the localization of enkephalin in striatopallidal neurons and substance P and dynorphin in striatonigral neurons, these lesion and pharmacologic data suggest that dopamine oppositely modulates neuronsc ontributing to the striatopallidal and striatonigral pathways. The differential localization of D1 and D2 dopamine receptors on striatonigral and striatopallidal neuronsprovides a reasonable explanation of the effect of dopamine on these neurons. The direct effect of D1 and D2 receptor activation has been studied with the unilateral 6-OHDA-lesioned animal as a model to examine regulation through D1 and D2 dopamine receptors (Gerfen et al 1990). With this paradigm, the regulation of D1 and D2 dopamine receptor, enkephalin, substance P, and dynorphin mRNA levels weree xamined in response to either intermittent (21 daily injections) or continuous (injections delivered through an osmotic minipump) systemic administration of D1 and D2 selective agonists. These studies show that reduced levels of substance P and D1 receptor mRNA levels resulting from 6-OHDA-induced nigrostriatal dopamine deafferentation are selectively reversed and dynorphin mRNA levels are sig nificantly elevated with intermittent treatments with the selective D1 agonist SKF-38393. Neither continuous SKF-38393 nor D2 selective agonist treatment affects levels of these mRNAs. Conversely, the elevation of both enkephalin and D2 receptor mRNA levels following 6-OHDA lesions is selectively reversed withc ontinuous treatment with the D2 selective agonist quinpirole (1mg/kg/day for 21 days). The other drug treatment regimens had no significant effect on these mRNA levels. These results provide direct evidence that dopamine differentially regulates gene expression in striatonigral and striatopallidal neurons through the respective selective expression of D1 and D2 dopamine receptors by these neurons. Moreover, the critical relationship between the time course of drug delivery and the selective action through the two receptors suggests that molecular processes by which receptor occupancy alters gene expression is fundamentally distinct for the two dopamine receptors. Studies employing D1 and D2 dopamine receptor agonisttreatments in intact animals have yielded more complex results, For example, D1 and not D2 antagonist treatments result in elevation of enkephalin levels (Jiang et al 1990), and D2 and not D1 agonist treatments have been reported to elevate substance P levels (Haverstick et al 1989). The differences between these results and those in the unilateral lesioned model may reflect interactions between subpopulations of neurons, which are discussed in detail below. Changes in peptide mRNA levels in striatonigral and striatopallidal neurons provide an excellent paradigm for examining receptor-specific mechanisms of dopaminergic regulation of these neurons. The functions of the specific peptides examined remain unknown, however. Moreover, as all of these neurons utilize GABA as a transmitter, the physiologic consequence of dopaminergic modulation of striatal neurons is probably mediated at least in part by GABA (Chevalier et al 1985). Rather, it is suggested that the specific changes in peptide levels provide a means of assaying the relative activity level of these neurons. Several lines of evidence would appear to substantiate making this assumption. First, it has been reported that D1 agonist treatments of 6-OHDA lesioned animals, which specifically elevate peptide levels in striatonigral neurons, also induce expression of the immediate early oncogene product c-fos (Robertson et al 1990). Second, studies employing comparable lesion and pharmacologic treatments as those used in examining peptide mRNA expression have revealed parallel changes in 2-deoxyglucose utilization in the globus pallidus and substantia nigra (Engber etal 1990, Trugman and Wooten 1987). Taken together, these studies suggest that dopamine deafferentation in the striatum results in both elevated enkephalin mRNA levels and increased activity in striatopallidal neurons (Figure 5). Furthermore, in lesioned animals D1 agonist treatments selectively increase substance P and dynorphin mRNA levels and activity in striatonigral neurons. Elevation of activity in the striatopallidal pathway resulting from striatal dopamine deafferentation has been proposed to underlie the bradykinesia of Parkinson's disease (Albin et al 1989, Mitchell et al 1989). Consistent with this are the concurrent decrease in 2-deoxyglucose utilization in the external pallido-subthalamic pathway and increased utilization in the internal pallido-thalamic pathway (Mitchell et al 1989). Bradykinesia is thus thought to be a result of increased activity in the indirect striatonigral output system, which results in increased tonic activity of the inhibitory inputs to the thalamus and brainstem targets of the internal segment of the globus pallidus and substantia nigra pars reticulata. Verification of this idea comes from the report that lesions of the subthalamic nucleus, designed to disrupt the indirect striatonigralpathway, result in profound reversal of bradykinesia in monkeys made Parkinsonian with MPTP lesions (Bergman et al 1990).
The Neostriatal Mosaic:
Multiple Levels of Compartmental Organization in the Basal Ganglia
Charles R. Gerfen
National Institute of Mental Health, Bethesda, Maryland 20892
Contents
General organization of the basal ganglia
Patch-matrix striatal compartments
Striatal patch-matrix output systems
Dopaminergic patch-matrix input systems
Corticostriatal patch-matrix inputs
Functional significance of patch-matrix compartments
Striataopallidal and striatonigral systems
Biochemical characterization of striatal ouput neurons
D1 and D2 dopamine receptor expression
Dopaminergic regulation of striatal output systems
Functional apsects of Parkinson's disease
Balanced opposition for striatopallidal and striatonigral outputs
D1 and D2 receptor interactions
D1 and D2 receptor expression in neurokinin B neurons
Local axon collalateral of medium spiny neurons
Striatal cholinergic interneurons
Patch-matrix compartments and striatopallidal and striatonigral systems
Conclusions
Summary, Acknowledgments, and References
Introduction
General Organizatin of the Basal Ganglia
Patch-Matrix Striatal Compartments
Striatal Patch-matrix Output Systems
Dopaminergic Patch-Matrix Input Systems
Corticostriatal Patch-Matrix Inputs
Functional Significance of Patch-Matrix Compartmentation
Striatopallidal and Striatonigral Systems
Biochemical Characterization of Striatal Output Neurons
Neurotransmitter and Peptide Content
D1 and D2 Dopamine Receptor Expression

Dopaminergic Regulation of Striatal Output Systems
Functional Aspects of Parkinson's Disease
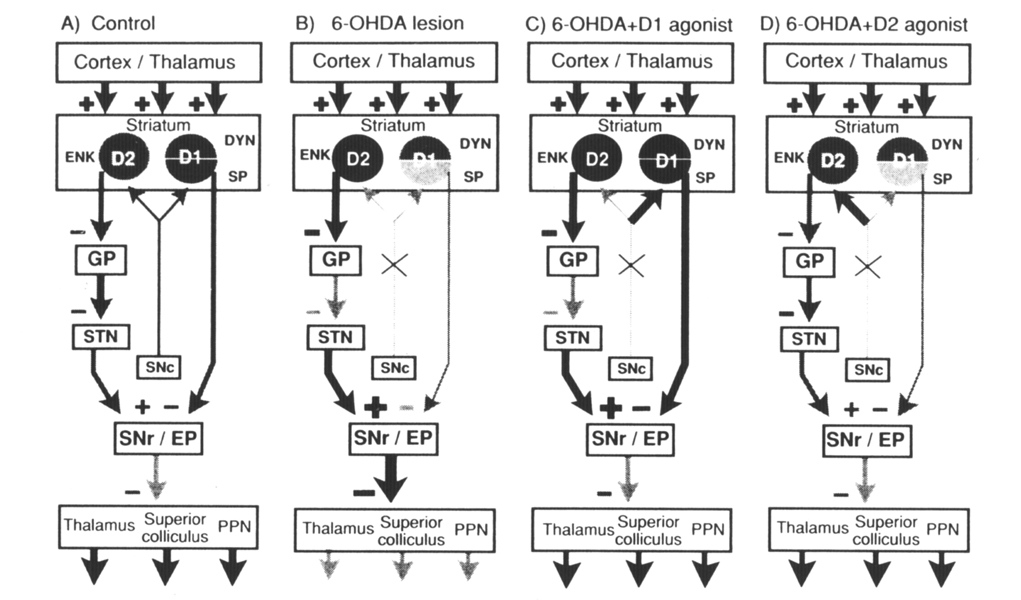
Figure 5. Diagram of the connections of striatal outputneurons. The grey level of the cells denotes relative peptide mRNA levels and the thickness of the lines indicates relative activity measured in studies with 2-deoxyglucose (Trugman and Wooten 1987). (A) Control: The cortex and thalamus provide excitatory input to the striatum. Striatal neurons that contain enkephalin (ENK) and the D2 dopamine receptor (D2) provide an inhibitory input to the globuspallidus (GP). Pallidal neurons inhibit the subthalamic nucleus (STN), which provides an excitatory input to the substantia nigra pars reticulata (SNr). Striatal neurons that express the D1 dopamine receptor (D1), dynorphin (DYN), and substance P (SP) provide an inhibitory input to the substantia nigra/entopeduncular nucleus (SNr/EP). SNr/EP GABAergic neurons inhibit neurons in the thalamus,superior colliculus, and pedunculopontine nucleus (PPN). Normal behavioral activity (arrows at the bottom of the diagram) is dependent on coordinated striatonigral and striatopallidal outputs that regulate SN output. (B) 6-OHDA: Dopamine lesions result in increased enkephalin expression and activity in striatopallidal neurons. This results in increased firing of SN GABAergic neurons and in diminished behavioral activity (arrows at bottom of diagram). (C) 6-OHDA+D1 agonist: D1 agonist treatment after 6-OHDA lesions does not alter the lesion-induced increase in enkephalin in the striatopallidal pathway but reverses the decrease in substance P and significantly increases dynorphin in striatonigral neurons. (D) 6-OHDA + D2 agonist: Continuous D2 agonist treatment after 6-OHDA lesions has no effect on the striatonigral pathway but reverses the lesion-induced increase in enkephalin in the striatopallidal pathway. This reverses the increased excitatory input from the subthalamic nucleus to the substantia nigra pars reticulata. From Gerfen et al (1990).
Therapeutic approaches to the treatment of Parkinson's disease might be suggested based on the understanding of D1 and D2 receptor regulation of striatal output pathways (Gerfen et al 1990). As discussed, the principal deficit- produced by striatal dopamine depletion in Parkinson's disease is an increase in striatopallidal activity, which is paralleled by increased enkephalin expression in striatopallidal neurons. To overcome this lesion induced increase in striatopallidal function, D2 receptor activation is necessary, and to be most effective appears to require a continuous treatment protocol with a D2 selective agonist. Supplemental treatment with a pharmacologic agent that acts on D1 receptors would be necessary to overcome the diminished function in this pathway caused by dopamine deafferentation. It is suggested, however, that the dosage required for a D1 agent would be less if given in conjunction with a D2 selective agonist. Interestingly, L-DOPA administered in twice daily injections to rats with 6-OHDA nigrostriatal lesions appears to affect striatonigral and not striatopallidal pathways in a manner similar to a D1 selective agonist (C. R. Gerfen and T. M. Engber, unpublished findings). Continuous treatment with L-DOPA appears to have little effect on either pathway. To have a behavioral effect, both D1 selective agonists and L-DOPA given on an intermittent schedule would appear to increase striatonigral function to an abnormally high level in order to overcome the increased function of striatopallidal neurons caused by dopamine deafferentation. Maintaining such abnormally high levels of function with long-term treatments might underlie the deterioration of L-DOPA as an effective therapeutic agent in the treatment of Parkinson's disease.
The disinhibitory processes of striatal outputs have provided a coherent model to explain the movement disorders that result from the loss of striatal dopamine in Parkinson's disease and animal models of this disease. Reversal of bradykinesia in MPTP-treated monkeys with lesions of the subthalamic nucleus has provided perhaps the best verification of this model (Bergman et al 1990). Extrapolating this model to the generation of normal behavior undoubtedly oversimplifies the processes involved. The generation of eye movements appears to be clearly related to disinhibitory processes whereby pauses in nigrotectal activity are correlated with eye movements (Hikosaka and Wurtz 1983a,b), yet single unit recording studies of internal globus pallidus activity in relationship to limb movements in primates reveals a more complex pattern of activity. In such studies, specific limb movements are correlated with both decreases and increases in the activity of internal pallidal firing, Such results are suggested to demonstrate that although disinhibitory mechanisms are involved in the generation of specific movements, the opposed mechanism, which results in increased inhibitory processes, may suppress antagonistic movements (Alexander and Crutcher 1990). These data are not incompatible with the model of Parkinson's disease discussed above but are introduced to illustrate the positive aspect that increased activity in the striatopallidal pathway may have in the generation of normal behavior. Thus, the balanced opposition in the relative activity of the striatopallidal and striatonigral pathways provides mechanisms for both allowing and disallowing specific muscle activity. In Parkinson's disease, the mechanisms for disallowing behavior appear to prevail, but in the normal condition, such processes are an integral part of the generation of normal behavior.
Evidence for the synergistic effects of D1 and D2 receptor activation is provided from the work of Walters and coworkers (Carlson et al 1990, Waiters et al 1987). Their studies have shown that D1 agonist pretreatment potentiates D2 agonist induced increases in globus pallidus activity (Walters et al 1987). Other studies have shown that in the 6-OHDA lesioned animal, combinations of D1 agonist and D2 agonist treatments result in a broader range of responses in pallidal neurons than occurs with treatments of only one of the agonists; some units show increases and some decreases in activity (Carlson et al 1990). Other studies have shown that both D1 and D2 receptor activation is required to inhibit the activity of (Na+ K+)ATPase in striatal neurons (Bertorello et al 1990). These latter studies suggest that synergistic effects of D1 and D2 receptor activation may occur within an individual neuron.
Based on current knowledge of the distribution of D1 and D2 receptors primarily in separate populations of striatal neurons, multiple mechanisms for the synergistic effect of D1 and D2 receptor activation are proposed. One mechanism may occur in neurons that coexpress D1 and D2 receptors, a second mechanism may, be mediated through the local axon collaterals that interconnect neurons that express different receptors, a third mechanism may involve striatal interneurons (Figure 6), and a fourth may involve the patch-matrix compartmental organization of the striatum.
The majority of striatal output neurons appear to express only one of the D1 and D2 dopamine receptors. Some striatal neurons may express both receptors, but another subset of output neurons, which contain the tachykinin neurokinin B, do not, for the most part, express either the D1 or D2 subtype (C. R. Gerfen, unpublished observations). Interestingly, neurokinin B mRNA levels are regulated by both D1 and D2 dopamine receptor mediated mechanisms. Nigrostriatal dopaminergic deafferentation results in an increase in neurokinin B mRNA, which is reversed with continuous treatment with the D2 selective agonist quinpirole (1 mg/kg/day for 5 days). The lesion-induced elevation of neurokinin B mRNA expression is further increased following intermittent D 1 selective agonist treatments (SKF-38393, 10 mg/kg/day for 5 days). Thus, these neurons represent an interesting subpopulation of striatal neurons in which effects of D1 and D2 dopamine receptor activation may occur within an individual neuron, but by indirect mechanisms.
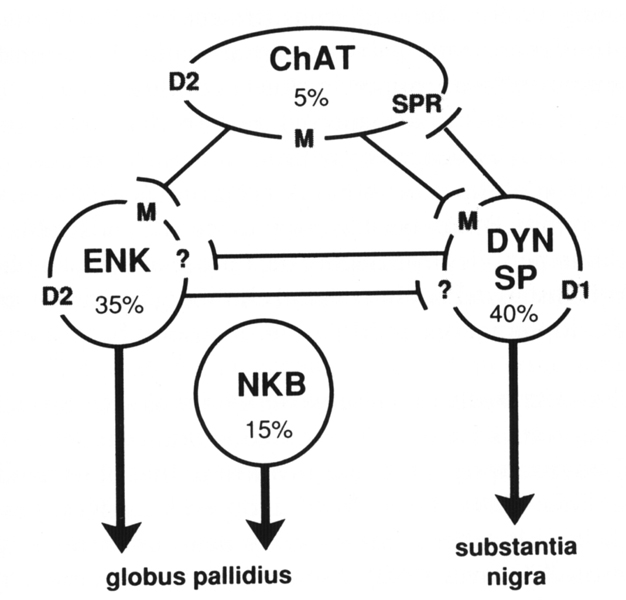
Figure 6. Schematic diagram of the major types of striatal neurons (approximate percentage of the total striatal population is listed) and the possible interactions between them that provide mechanisms for balancing striatopallidal and striatonigral outputs. Cholinergic interneurons (ChAT) express the D2 dopamine-, muscarinic-, and substance P receptor (SPR) mRNAs. Striatonigral neurons contain the peptides dynorphin (DYN) and substance P (SP) and express the D1 dopamine and muscarinic (M) receptor mRNAs.
The majority of striatopallidal neurons contain enkephalin (ENK) and express the D2 dopamine- and muscarinic receptor mRNAs. A minority of striatopallidal neurons contain neurokinin B (NKB) and do not express either the D1 or D2 dopamine receptor mRNAs. Interactions between D1 and D2 receptors may occur in NKB neurons by direct interactions between ENK and DYN neurons through an unknown receptor mechanism, or via substance P mediated regulation of cholinergic neurons.
Intrastriatal connections of the NKB neurons are still relatively unknown. Neurokinin B is contained in 15–20% of striatal output neurons (Burgunder and Young 1989). These neurons project principally to the globus pallidus and thus represent a subset of striatopallidal neurons. Although some of these neurons are grouped in clusters of three to five, for the most part they are distributed in a dispersed pattern that does not show any clear grouping such as evidenced by neurons that form striatal patches. The cellular localization of the neurokinin B receptor has yet to be determined; however, neurokinin B has been shown to be the most effective of the striatal tachykinin peptides in inducing the release of acetylcholine (Arenas et al 1991). As is discussed below,striatal cholinergic neurons are critically involved in the dopaminergic modulation of striatal output neurons.Striatal medium spiny output neurons have a local axon collateral that for most neurons spreads in a domain approximately equivalent to that of its dendritic arbor (Wilson and Groves 1980). These local axon collaterals appear to make synaptic contact with other medium spiny neurons through asymmetric synapses (Wilson and Groves 1980). As mentioned above, these neurons use GABA as a neurotransmitter and also express GABA and benzodiazepine receptor mRNAs (Lolait et al 1989). Thus, neighboring striatal output neurons are connected in a manner, and express the appropriate receptors, that may provide for their affecting one another through a GABA mediated inhibitory synaptic process (Park et al 1980). This is one potential mechanism whereby a striatonigral neuron that is specifically modulated through its expression of a D1 receptor might influence the activity of a neighboring striatopallidal neuron that expresses a D2 receptor. Such mechanisms are still only postulated, as there is as yet no direct evidence for such interactions. The peptides that are co-expressed by these neurons also are potential neuroactive substances through which such interactions may occur. For the most part, however, the localization of receptors to which the opiate peptides bind has not been determined, so the role of these peptides is unclear.
The substance P receptor, a G-protein-coupled receptor that has recently been cloned and characterized (Hershey and Krause 1990, Yokota etal 1989), is localized specifically on striatal cholinergic neurons (Gerfen 1991b). Because substance-P-containing local axon collaterals of medium spiny neurons make synaptic contact with cholinergic neurons (Bolam et al 1986), a specific mechanism whereby cholinergic neurons are involved in interactions between striatal output neurons is suggested. This is discussed below.
A small number of striatal neurons have large cell bodies that extend aspiny dendrites over relatively large domains (up to 1000 microns in extent) and extend an even more widespread axon collateral that remains confined to the striatum (Wilson et al 1990). These aspiny interneurons express choline acetyltransferase and are referred to as striatal cholinergic interneurons. These neurons are distinct from other striatal neurons in exhibiting irregular but tonic activity (Wilson et al 1990). Although the neurons are relatively sparse, representing less than 5% of the striatal population in rats, they exert a profound influence on striatal function. The mechanisms of this influence are complex and are now being made clear with studies elucidating the mechanisms that regulate acetylcholine release and the post-synaptic action of acetylcholine through muscarinic receptors expressed on striatal neurons.
Dopamine appears to inhibit acetylcholine release through a D2-receptor-mediated process (Lehmann and Langer 1983 , Stoofand Kebabian 1982) that is modulated in part by muscarinic autoreceptors expressed on cholinergic neurons (Drukarch et al 1990). Increased acetylcholine release that follows dopamine deafferentation of the striatum may be partly responsible for the increased activity of striatopallidal neurons. This is supported by the finding that the muscarinic antagonist scopolamine partially blocks neuroleptic-induced elevation of striatal enkephalin levels (Hong et a] 1985). Subtypes of muscarinic cholinergic G-protein-coupled receptors have been cloned (Bonner et al 1987). In situ hybridization localization has shown that the m1 subtype isexpressed by most striatal projection neurons, the m2 subtype isselectively expressed by striatal cholinergic neurons, and the m4subtype is expressed by approximately half of the striatal outputneurons (Weiner et al 1990). The functional significance of thedifferential pattern of muscarinic receptor subtype distribution inthe striatum is not yet clear; however, their localization does place the subtypes in a position to mediate cholinergic regulation ofstriatal output neurons. The possible complexity of this regulationis suggested by a recent study that showed the physiologic effect of muscarinic receptor activation, mediated through a K+ channel, to depend on the state of the membrane polarization of the post-synaptic neuron (Akins et al 1990). Akins et al propose that the effect of muscarinic receptor activation is to stabilize the resting membrane potential of medium spiny neurons, which normally fluctuates between two levels of hyperpolarization (Wilson 1981).
The tachykinin peptides neurokinin B, substance K, and substance P have been shownto induce release of acetylcholine in the striatum (Arenas et al1991). This raises the possibility that a functional role of these peptides, which are expressed by different sets of striatal output neurons, may be to regulate the release of acetylcholine. Such a function is dependent on the localization of the appropriate tachykinin receptors. In this context it is of interest that the substance P receptor (neurokinin 1 receptor) is selectively localized to the cholinergic neurons in the striatum and does not appear to be expressed by other striatal neurons (Gerfen 1991). This suggests another possible mechanism for the interaction between D1 and D2 dopamine receptors. Stimulation of D1 receptors appears to increase substance P expression and may increase its release, which, acting on substance P receptors on cholinergic neurons, could increase the release of acetylcholine. An increase in acetylcholine release may then affect striatopallidal neurons, whose response to muscarinic receptor activation is modulated by D2 dopamine mechanisms (C. R.Gerfen , unpublished observations). At this time a mechanism can only be postulated, but it is suggested to illustrate a possible intercellular mechanism that may underlie interaction between D1 and D2 dopamine receptors.
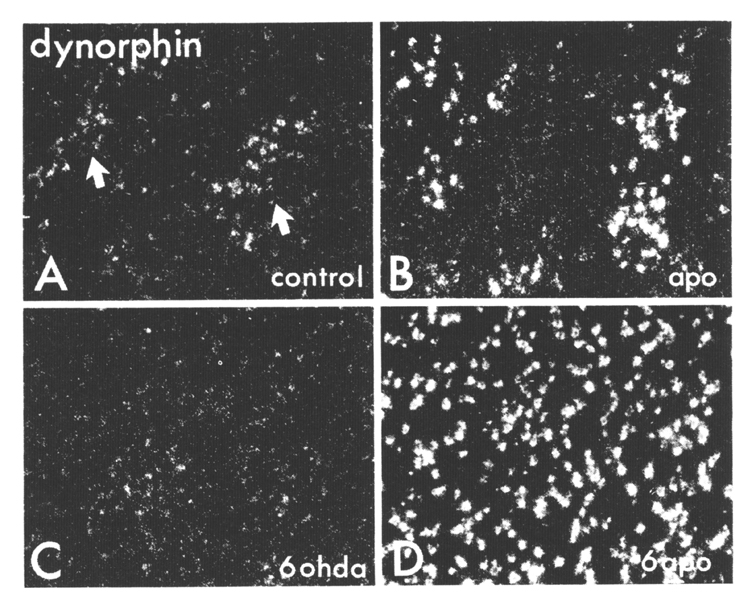
Figure 7. Darkfield photomicrographs ofautoradiographically generated grains (seen as white dots) producedby ISHH labeling of striatal sections via an oligonucleotide probe complementary to dynorphin rnRNA (A-D). Sample areas in the dorsolateral striatum are shown from a control animal (A), from an animal that received 10 days of twice daily injections of 5 mg/kg apormorphine (B), from the lesioned side of an animal that had a 6OHDA lesion of the nigrostriatal pathway (C), and from the lesioned side of an animal that received a 6-OHD. A lesion followed 7 days later by 10 days of twice daily apornorphine treatments (D). Note that in the controls, neurons in the patches (indicated with arrows and identified in adjacent sections with 3H-naloxone binding, not shown) show more labeling than do cells in the matrix. From Gerfen et al (1991).
Although the functional role of the selective expression of the substance P receptor by cholinergic neurons remains speculative, it is appropriate to comment on the possible significance of the role of peptides in the basal ganglia. Substance P is expressed by striatonigral neurons and is localized in terminals in the substantia nigra by immunohistochemical techniques (Haber and Nauta 1983), yet substance P receptor binding studies have failed to demonstrate appropriate binding sites within the substantia nigra (Danks et al 1986). This has been cited as one of numerous cases of a mismatch between receptor binding sites and the localization of endogenous ligands (Herkenham 1987). Striatonigral neurons also utilize GABA as a transmitter, and for this there is evidence of a physiologic role in that stimulation of striatal outputs results in GABA-mediated inhibition of neurons in the substantia nigra (Chevalier et al 1985). Thus, striatonigral neurons may affect striatal medium spiny neurons and substantia nigra neurons by a GABA receptor mediated process and simultaneously induce release of acetylcholine from striatal cholinergic neurons by a substance P receptor mediated process. This suggests that receptor binding and endogenous ligand mismatches may reflect the synthesis and transport of a specific neuropeptide in all axon collaterals of a neuron but that the actual physiologic action of that peptide may occur only at those synaptic sites at which the postsynaptic neuron expresses the appropriate receptor. This organization provides a neuron with the capability of differentially affecting different target neurons dependent on the type of receptor expressed by those target neurons.
The subpopulations of striatal output neurons described above, namely striatopallidal neurons that express enkephalin and the D2 dopamine receptor, and striatonigral neurons that express substance P, dynorphin, and the D1 dopamine receptor, appear to be evenly distributed in both patch and matrix compartments (Gerfen and Young 1988). Nonetheless, the relative expression of some of these markers in individual neurons appears to be different in the patch-matrix compartments. For example, substance P mRNA levels appear to be higher in patch than in matrix neurons in the ventral striatum, whereas dynorphin mRNA levels appear to be higher in patch than in matrix neurons in the dorsal striatum (Gerfen and Young 1988). That these patterns of relative expression are under dopaminergic regulation is suggested by the findings that in the intact striatum, dopamine agonist treatments elevate dynorphin in dorsal and substance P in ventral patch neurons, somewhat specifically (Gerfen et al 1991, Li et al 1987, 1988).
The dopaminergic regulation of the expression of dynorphin in the dorsal striatum may serve as a model of the functional significance of the patch-matrix compartments as they relate to the regulation of the balanced opposition of striatopallidal and striatonigral output systems (Figure 7, Gerfen et al 1991). In the normal striatum,complex interactions may occur between subpopulations of striatal output neurons and interneurons that establish a balance in the regulation of the two output systems. The pattern of expression of dynorphin mRNA in the dorsal striatum delineates these interactions. Dynorphin is expressed by equal numbers of patch and matrix neurons, but its expression in patch neurons is relatively higher than in matrix neurons. In rats treated with the nonselective dopamine agonist apomorphine, this relative difference in dynorphin expression in the patch compartment is augmented. In the dopamine deafferented striatum, however, the same apomorphine treatment or D1 selective agonist treatment results in a dramatic elevation of dynorphin mRNA expression in both patch and matrix neurons. In this situation, the normal regulatory mechanisms that establish a balance in the striatonigral and striatopallidal output systems may become inoperative. In addition to the possible cellular and molecular mechanisms regulating the balanced opposition of these output systems discussed above, several relating to patch-matrix compartmental organization may also be involved. One is the dual mesostriatal dopaminergic system originating from dorsal and ventral tier dopamine neurons that differentially targets the matrix and patch compartments, respectively (Gerfen et al 1987). In the normal animal, these systems may provide some mechanism of differentially altering peptide expression in the striatum. Another is the possible role of a striatal interneuronal system. Somatostat in striatal interneurons distribute fibers that are somewhat restricted to the matrix compartment (Chesselet and Graybiel 1985 , Gerfen 1984 , 1985) and could mediate the differential effect on peptides in the compartments via a dopaminergic mediated process (Chesselet and Reisine 1983). Unfortunately, we can still only speculate as to the regulatory mechanisms involved. Nonetheless, patch-matrix compartmental organization appears to provide mechanisms for differentially regulating striatopallidal and striatonigral output neurons, and these influences appear to vary regionally.
Balanced opposition in the cortically driven activity of striatopallidal and striatonigral pathways is proposed to underlie the complex manner in which the basal ganglia affect behavior. This review has described aspects of the compartmental organization of the striatum that provide both cellular and molecular mechanisms responsible for regulating this balance. Although all striatal neurons utilize GABA as a transmitter, the majority of striatopallidal neurons express the D2 dopamine receptor and enkephalin, whereas the majority of striatonigral neurons express the D1 dopamine receptor and the peptides dynorphin and substance P. In the dopamine depleted striatum, D2 agonists decrease and D1 agonists increase peptide expression in striatopallidal and striatonigral neurons, respectively. This suggests that the direct action of dopamine is to oppositely modulate these output pathways; however, the rather uniform responses of striatonigral and striatopallidal neurons would appear to occur on the unique condition that one dopamine receptor subtype is activated in the absence of the other.
In the normal striatum, intra- and intercellular interactions occur that provide for more complex patterns of regulation of striatonigral and striatopallidal outputs. Four such mechanisms of D1 and D2 receptor interaction are described. One type occurs in a subpopulation of striatopallidal neurons that express both D1 and D2. A second mechanism of interaction may occur by way of the local axon collaterals of medium spiny neurons that may interconnect neurons that express different dopamine receptors. A third mechanism may involve striatal interneurons. Expression of the tachykinin substance P in striatonigral neurons is elevated by D1 agonist stimulation. Striatal cholinergic interneurons express the substance P receptor and release acetylcholine in response to its activation. Since cholinergic muscarinic receptor activation regulates striatopallidal neurons, a cholinergic mediated linkage of striatonigral and striatopallidal neurons is proposed. A fourth mechanism of regulation of striatonigral and striatopallidal output neurons may involve the patch- matrix compartmental organization of the striatum. Peptide expression in these output neurons varies regionally in patch-matrixc ompartments in a manner that suggests dopaminergic and possibly striatal interneuronal involvement. These mechanisms of D1 and D2 receptor interactions are not exhaustive but are presented to illustrate possible mechanisms for the generation of complex patterns of regulation of the balanced opposition of striatonigral and striatopallidal outputs.
In conclusion, perhaps the fundamental function of the basal ganglia is to select from myriad possibilities a specific behavioral action. Balanced opposition of the activity of striatonigral and striatopallidal neurons provides the mechanism whereby a specific behavior is facilitated or disfacilitated. Studies examining gene regulation of subpopulations of striatal output neurons reveal the cellular and molecular mechanisms involved in this process. The critical processes whereby select subpopulations of striatal neurons are coordinated are dependent on the integrative properties of corticostriatal systems (Wilson 1990) that interact with the intrastriatal organization of mechanisms regulating the balanced opposition of striatonigral and striatopallidal output systems.
ACKNOWLEDGMENTS
I would like to thank Charlie Wilson, Miles Herkenham, Lennart Heimer, Gary Alexander, Roger Albin, Mahlon DeLong, and Steve Wise for discussions during the course of writing this review. This work was supported by the Intramural Research Program of the National Institute of Mental Health.
Literature Cited
Akins, P. T., Surmeier, D. J., Kitai, S.T. 1990. Muscarinic modulation of a transient K+ conductance in rat neostriatal neurons, Nature 344: 240–242
Albin, R. L., Young, A. B., Penney, J. B. 1989. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12: 366–375
Alexander, G. E., Ctutcher, M. D. 1990. Functional architecture of basal ganglia circuits: Neural substrates of parallel processing, Trends Neurosci. 13: 266–271
Alexander, G. E., DeLong, M. R., Strick, P.L. 1986 Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev. Neurosci. 9: 357–381
Alheid, G. F., Heimer, L. 1988. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticipetal components of the substantia innominata. Neuroscience 27: 1–39
Arenas, E., Alberch, J., Perez-Navarro, E., Solsona, C., Marsal, J. 1991. Neurokinin receptors differentially mediate endogenous acetylcholine release evoked by tachykinins in the neostriatum. J. Neurosci. 11: 2332–2338
Bannon, M. J., Lee, J.-M., Girand, P., Young, A., Affolter, J.-U., et al. 1986. Dopamine antagonist baloperidol decreases substance P, substance K and preprotachykinin mRNAs in rat striatonigral neurons. J. Biol. Chem. 261: 6640–6642
Beckstead, R. M. 1988, Association of dopamine D1 and D2 receptors with specific cellular elements in the basal ganglia of the cat: The uneven topography of dopamine receptors in the striatum is determined by intrinsic striatal cells, not nigrostriatal axons, Neuroscience 27: 851–863
Beckstead, R. M., Kersey, K. S. 1985. Immunohistochemical demonstration of differential substance P-, Met-enkephalin-, and glutamic acid decarboxylase-containing cell and axon distributions in the corpus striatum of the cat. J. Comp Neurol. 232: 481–498
Bergman, H., Whitman, T., DeLong, M. R. 1990. Reversal of experimental Parkinsonism by lesions of the subthalamic nucleus, Science 249: 1436–1438
Bertorello, A. M., Hopfield, J. F., Aperia, A., Greengard, P. 1990. Inhibition by dopamine of (Na+ + K+)ATPase activity in neostriatal neurons through D1 and D2 dopamine receptor synergism. Nature 347: 386–388
Bolam, J. P., Ingham, C. A., Izzo, P. N., Levey, A. I., Rye, D. B., et al. 1986. Substance P–containing terminals in synaptic contact with cholinergic neurons in the neostriatum and basal forebrain: A double immunocytochemical study in the rat. Brain Res. 397: 279–289
Bolam, J. P., Izzo, I. N., Graybiel, A. M. 1988. Cellular substrate of the histochemically defined striosome/matrix system of the caudate nucleus: A combined Golgi and immunocytochemical study in cat and ferret. Neuroscience 24. 853–875
Bolam, J. P., Wainer, B. H., Smith, A. D. 1984. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi–impregnation and electron microscopy. Neuroscience 12: 711–712
Bonner, T. I., Buckley, N. J., Young A.C., Brann, M. R. 1987. Identification of a family of muscarinic acetylcholine receptor genes. Science 237: 527–532
Bouyer, J. J. , Park, D. H., Joh, T. H., Pickel, V. M. 1984. Chemical and structural analysis of the relation between cortical inputs and tyrosine hydroxylase-containing terminals in rat neostriatum. Brain Res. 302: 267–275
Bunzow, J. R., Tol, H. H. M. V., Grandy, D. K., Albert, P., Salon, J., et al. 1988. Cloning and expression of a rat D2 dopamine receptor. Nature 336: 783–787
Burgunder, J. M., Young, W. S. 1989 Distribution, projection and dopaminergic regulation of the neurokinin B mRNA-containing neurons of the rat caudate-putamen. Neuroscience 32: 323–335
Carman, J. B., Cowan, W. M., Powell, T. P. S. 1965. The organization of corticostriate connexions in the rabbit. Brain 86: 525–562
Carlson, J. H., Bergstrom, D. A., Demo, S D., Walters, J. R. 1990. Nigrostriatal lesion alters neurophysiological responses to selective and nonselective Dl and D2 dopamine agonists in rat globus pallidus. Synapse 5: 83–93
Cheramy, A., Leviel, V., Glowinski, J. 1981. Dendritic release of dopamine in the substantia nigra. Nature 289: 537–542
Chesselet, M.-F., Graybiel, A. M. 1985. Striatal neurons expressing somatostatin-like immunoreactivity: Evidence for a peptidergic interneuronal system in the cat. Neuroscience 17: 547–571
Chesselet, M.-F., Reisine, T. D. 1983. Somatostatin regulates dopamine release in striatal slices and cat caudate nuclei. J. Neurosci. 3: 232–236
Chevalier, G., Vacher, S., Deniau, J. M., Desban, M. 1985. Disinhibition as a basic process in the expression of striatal function. I. The striatonigral influence on tecto-spinal/tecto-diencephalic neurons, Brain Res. 334: 215–226
Cowan, R. C., Wilson, C. J., Emson, P. C., Heizmann, C. W. 1990. Parvalbumin containing GABAergic interneurons in the rat neostriatum. J. Comp. Neurol. 302: 197–205
Danks, J. A., Rothman, R. B., Cascieri, M. A., Chicchi, G. G., Liang, T., et al. 1986. A comparative autoradiographic study of the distribution of substance P and eledoisin binding sites in rat brain. Brain Res. 385: 273–281
Dearry, A., Gingrich, J. A., Faladrdeau, P., Fremeau, J. R. T., Bates, M. D., et al. 1990. Molecular cloning and expression of the gene for a human D1 dopamine receptor. Nature 347: 72–76
Deniau, J. M., Chevalier, G. 1985. Disinhibition as a basic process in the expression of striatal functions. II. The striato-nigral influence on thalamocortical cells of the ventromedial thalamic nucleus. Brain Res, 334: 227–233
DiFiglia, M., Aronin, N. 1982. Ultrastructural features of immunoreactive somatostatin neurons in the rat caudate nucleus, J. Neurosci. 2: 1267–1274
Donoghue, J. P., Herkenham, M. 1986. Neostriatal projections from individual cortical fields conform to histochemically distinct striatal compartments in the rat. Brain Res. 365: 397–403
Donoghue, J. P., Kitai, S. T. 1981. A collateral pathway to the neostriatum from corticofugal neurons of the rat sensory-motor cortex: An intracellular HRP study. J. Comp. Neurol. 210: 1–13
Drukarch, B., Schepens E., Stoof, J. C. 1990. Muscarinic receptor activation attenuates D2 dopamine receptor mediated inhibition of acetylcholine release in rat striatum: Indications for a common signal transduction pathway. Neuroscience 37: 1–9
Engber, T. M., Susel, Z., Kuo, S., Chase, T. N. 1990. Chronic levodopa treatment alters basal and dopamine agonist-stimulated cerebral glucose utilization. J. Neurosci. 10: 3889–3895
Felder, C. C., Jose, P. A., Axelrod, J. 1989. The dopamine-1 agonist, SKF 82526, stimulates phospholipase-C activity independent of adenylate cyclase. J. Pharmacal. Exp. Ther. 248: 171–175
Ferino, F., Thierry, A. M., Saffroy, M., Glowinski, J. 1987. Interhemispheric and subcortical collaterals of medial prefrontal cortical neurons in the rat. Brain Res.417:257–266
Freund, T. F., Powell, J. F., Smith, A. D, 1984. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons with particular reference to dendritic spines. Neuroscience 13: 1189–1215
Gerfen, C. R. 1984. The neostriatal mosaic: Compartmentalization of corticostriatal input and striatonigral output systems. Nature 311: 461–464
Gerfen, C. R. 1985. The neostriatal mosaic: I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J. Comp. Neurol. 236:454–476
Gerfen, C. R. 1989. The neostriatal mosaic: Striatal patch-matrix organization is related to cortical lamination. Science 246: 385–388
Gerfen, C. R. 1991. Substance P receptor mRNA selectively expressed by cholinergic neurons in the striatum and basal forebrain. Brain Res. 556: 165–170
Gerfen, C. R., Baimbridge, K. G., Miller, J.J. 1985. The neostriatal mosaic: Compartmental distribution of calcium binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proc. Natl. Acad. Sci. USA 82: 8780–8784.
Gerfen, C. R., Baimbridge, K. G., Thibault, J. 1987a. The neostriatal mosaic. III. Biochemical and developmental dissociation of dual nigrostriatal dopaminergic systems. J. Neurosci. 7: 3935–3944
Gerfen, C. R., Engber, T. M., Mahan, L. C., Susel, Z., Chase, T. N., et al. 1990. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons, Science 250: 1429–1432
Gerfen, C. R., Herkenham, M., Thibault, J. 1987b. The neostriatal mosaic. II. Patch– and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J. Neurosci. 7: 3915–3934
Gerfen, C. R., McGinty, J. F., Young, I. W.S. 1991. Dopamine differentially regulates dynorphin, substance P and enkephalin expression in striatal neurons: In situ hybridization histochemical analysis. J. Neurosci. 11: 1016–1031
Gerfen, C. R., Sawchenko, P. E. 1984. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: Immunohistochemical localization of an axonally transported plant lectin. Phaseolus vulgaris-leucoaglutin (PHA-L). Brain Res. 290: 219–238
Gerfen, C. R., Staines, W. A., Arbuthnott, G, W., Fibiger, H. C. 1982. Crossed connections of the substantia nigra in the rat. J. Comp. Neural. 207: 283–303
Gerfen, C, R., Young, W. S. 1988. Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: An in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 460:161–167
Gilbert, C. D., Kelly, J. P. 1975. The projection of cell in different layers of the cat's visual cortex. J. Comp. Neurol. 163: 81–105
Gimenez-Amaya, J. M.,
Graybiel, A. M. 1990. Compartmental origins of the striatopallidal projections in the primate. Neuroscience 34: 111–126
Goldman-Rakic, P. S. 1982. Cytoarchitectonic heterogeneity of the primates neostriatum: Subdivision into island and matrix cellular compartments. J. Comp. Neurol. 205: 398–413
Gonzalez C., Chesselet, M.-F. 1990. Amygdalonigral pathway: An anterograde study in the rat with phaseolus vulgaris leucogglutinin (PHA-L) J. Comp. Neurol. 297: 182–200
Graybiel, A. M., Ragsdale, J. C. W. 1978. Histochemically distinct compartments in the striatum of human, monkey and cat demonstrated by acetylcholinesterase staining. Proc. Nati. Acad. Sci. USA 75: 5723–5726
Graybiel, A. M., Chesselet, M. -F. 1994. Compartmental distribution of striatal cell bodies expressing [Met]enkephalinlike immunoreactivity. Proc. Natl. Acad. Sci. USA 81: 7980–7984
Graybiel, A. M., Ragsdale, J. C. W., Yoneika, E, S., Elde, R. P. 1981. An immunohistochemical study of enkephalins and other neuropeptides in the striatum of the cat with evidence that the opiate peptides are arranged to form mosaic patterns in register with the striosomal compartments visible with acetylcholinesterase staining. Neuroscience 6: 377–397
Grofova, I. 1975. The identification of striatal and pallidal neurons projecting to substantia nigra. An experimental study by means of retrograde axonal transport of horseradish peroxidase. Brain Res. 91: 286–291
Grove, E. A. 1988. Efferent connections of the substantia innominata in the rat. J. Comp. Neurol. 277: 347–364.
Grove, E. A., Domesick, V. B., Nauta, W.J.H. 1986 Light microscopic evidence of striatal input to intrapallidal neurons of cholinergic cell group Ch4 in the rat: A study employing the anterograde tracer Phaseolus vulgaris leucoagglutinin (PHAL). Brain Res. 367: 379–84
Haber, S. N., Groenewegen, H. J., Grove, E.A., Nauta, W. J. H. 1985. Efferent connections of the ventral pallidum: Evidence of a dual striato pallidofugal pathway. J. Comp. Neurol. 235: 322–35
Haber, S. N., Nauta, W. J. H. 1983. Ramifications of the globus pallidus in the rat as demonstrated by patterns of immunohistochemistry. Neuroscience 9: 245–60
Hallanger, A. E., Levey, A. I., Lee, H. J., Rye, D. B., Wainer, B. H. 1987. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J. Comp Neurol. 262:105–24
Hanson, G. R., Alphs, L., Pradham, S., Lovenberg, W. 1981a. Haloperidol induced reduction of nigral substance P like immunoreactivity: A probe for the interactions between dopamine and substance P neuronal systems. J. Pharmacal. Exp. Ther. 218: 568–74
Hanson, G. R., Alphs, L., Pradham, S., Lovenberg, W. 1981b. Response Of striatonigral substance P systems to a dopamine receptor agonist and antagonist. Neuropharmacology 20: 541–48
Harrison, M. B., Wiley, R. G., Wooten, G.F. 1990. Selective localization of striatal Di receptors to striatonigral neurons. Brain Res. 528: 317–22
Hattori, T., McGeer, E. G., McGeer, P. L. 1978. Fine structural analysis of corticostriatal pathway. J. Comp. Neurol. 185: 347–54
Haverstick, D. M., Rubenstein, A., Bannon, M.J. 1989. Striatal tachykinin gene expression regulated by interaction of Dl and D2 dopamine receptors. J. Pharmacal. Exp Ther. 248: 858–62
Heimer, L., Wilson, R. D. 1975. The subcortical projections of the allocortex: Similarities in the neural associations of the hippocampus, the piriform cortex, and the neocortex. In Golgi Centennial Symposium, ed. M Santini, pp/ 177–93. New York: Raven
Herkenham, M. 1986. New perspectives on the organization and evolution of nonspecific thalamocortical projections. In Cerebral Cortex, ed. E. G. Jones, A. Peters, pp. 403–45. New York: Plenum
Herkenham, M. 1987. Mismatches between neurotransmitter and receptor localizations in brain: Observations and implica tions. Neuroscience 23: 1–38
Herkenham, M, Edley, S. M., Stuart, J. 1984. Cell clusters in the nucleus accumbens of the rat, and the mosaic relationship of opiate receptors, acetylcholinesterase and subcortical afferent terminations. Neuroscience II: 561–93
Herkenham, M., Pert, C. B., 1981. Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat striatum. Mature 291: 415–18
Hershey, A. D., Krause, J. E. 1990. Molecular characterization of a functional cDNA encoding the rat substance P recep tor. Science 247: 958–62
Hikosaka, O., Wurtz, R. H. 1983a. Visual and occulomotor functions of monkey substantia nigra pars reticulata. Ill. Memory contingent visual and saccade responses. J. Neurophysiol 49: 1268–84
Hikosaka, O., Wurtz, R. H. 1983b. Visual and occulomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J. Neurophysiol. 49: 1285–1301
Hong, J. S, Tilson, H. A., Yoshikawa, K. 1983. Effects of lithium and haloperidol administration on the rat brain levels of substance P. J. Pharmacal. Exp. Ther. 224: 590
Hong, J. S., Yang, H.-Y. T., Fratta, W., Costa, E. 1978. Rat striatal methionine-enkephalin content after chronic treatment with cataleptogenic and noncataleptogenic drugs. J. Pharmacal. Exp. Ther. 205: 141–47
Hong, J. S., Yoshikawa, K., Kanamatsu, T., Sabol, S. L. 1985. Modulation of striatal enkephalinergic neurons by antipsychotic drugs. Fed. Proc. 44: 2535–39
Hornykiewicz, O. 1966. Dopamine (3-hydroxytryptamine) and brain function. Pharmacal. Rev. 18: 925–64
Jiang, H.-K., McGinty, J. F., Hong, J. S. 1990. Differential modulation of striatonigral dynorphin and enkephalin by dopa, mine receptor subtypes. Brain Res. 507: 57–64
Jimenez-Castellanos, J., Graybiel, A. M. 1987. Subdivisions of the dopamine-containing A8-A9-A10 complex identified by their differential mesostriatal innervation of striosomes and extrastriosomal matrix. Neuroscience 23: 223–242
Jimenez-Castellanos, J., Graybiel, A. M. 1989. Compartmental origins of striatal efferent projections in the cat. Neuro science 32: 297–321
Jinnai, K., Matsuda, Y. 1979. Neurons of the motor cortex projecting commonly on the caudate nucleus and the lower brainstem in the cat. Neurosci. Lett. 13: 121–26
Jones, E. G. 1984. Laminar distribution of cortical efferent cells. In Cerebral Cortex, Vol. 1: Cellular Components of the Cerebral Cortex, ed. Jones, E. G., pp. 521–553, New York: Plenum
Jones, E. G., Coulter, J. D., Burton, H., Porter, R. 1977. Cells of origin and terminal distribution of corticostriatal fibers arising in the sensory-motor cortex of monkeys. J. Comp. Neurol. 173: 53–80
Kawaguchi, Y., Wilson, C. J., Emson, P. 1989. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J. Neurophysiol 62. 1052–68
Kawaguchi, Y., Wilson, C. J., Emson, P. 1990. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J. Neurosci. 10: 3421–38
Kebabian, J. W., Calne, D. B. 1979 Multiple receptors for dopamine. Nature 277: 9396
Kemp, J. M., Powell, T. P. S. 1971. The structure of the caudate nucleus of the cat: Light and electron microscopic study. Philos. Trans. R. Sac. London (Biol.) 262: 383–401
Kemp, J. M., Powell, T. P. S. 1970. The cortico-striate projection in the monkey. Brain 93: 525-46
Kita, H., Chang, H. T., Kitai, S. T. 1983. Pallidal inputs to subthalamus: Intracellular analysis. Brain Res. 264: 255–65
Kita, H. , Kitai, S. T. 1987a. Efferent projections of the subthalamic nucleus in the rat: Light and electron microscopic analysis with the PHA-L method. J. Comp. Neurol. 260: 435–52
Kita, H., Kitai, S. T. 1987b. Subthalamic inputs to the globus pallidus and the substantia nigra in the rat: Light and electron microscopic studies using PHA-L methods. J. Comp. Neurol. 260:435–52
Kita, H., Kitai, S. T. 1988. Glutamate decarboxylase immunoreactive neurons in rat neostriatum: Their morphological types and populations. Brain Res. 447: 346–52
Kitai, S. T., Koscis, J. D., Preston, R. J., Sugimori, M. 1976. Monosynaptic inputs to caudate neurons identified by intracellular injection of horseradish peroxidase. Brain Res. 109: 601–6
Krettek, J. E., Price, J. L. 1977. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J. Comp. Neurol. 171: 157–92
Le Moine, C., Normand, E., Guitteny, A. F., Fouque, B., Teoule, R., et al. 1990. Dopamine receptor gene expression by enkephalin neurons in rat forebrain. Proc. Natl. Acad. Sci. USA 87: 230–34
Lehmann, J., Langer, S. Z. 1983. The striatal cholinergic interneuron: Synaptic target of dopaminergic terminals? Neuroscience 10: 1105–20
Li, S. J., Sivam, S. P., McGinty, J. F., Douglass, J., Calavetta, L., et al. 1988. Regulation of the metabolism of striatal dynorphin by the dopaminergic system. J. Pharmacal. Exp. Ther. 246: 403–8
Li, S. J., Sivam, S. P., McGinty, J. F., Huang, Y.S., Hong, J. S. 1987. Dopaminergic regulation of tachykinin metabolism in the striatonigral pathway. J. Pharmacal. Exp. Ther. 243: 792–98
Lolait, S. J., O'Carroll, A.–M., Kusano, K., Mahan, L, C. 1989. Pharmacological characterization and region-specific expression in brain of the B2- and B3-subunits of the rat GABA(A) receptor. FEBS Lett. 258: 17–21
Loopuijt, L, D., van der Kooy, D. 1985. Organization of the striatum: Collateralization of its efferent axons. Brain Res. 348: 86–99
Mahan, L. C. Burch, R. M., Monsma, J.F.J., Sibley, D. R. 1990. Expression of striatal D1 dopamine receptors coupled to inositol phosphate production and Ca++ mobilization in xenopus oocytes. Proc. Natl. Acad. Sci. USA 87– 2196–2200
Malach, R., Graybiel, A. M. 1986. Mosaic architecture of the somatic sensory–recipient sector of the cat's striatum. J. Neurosci. 6. 3436–58
McKinney, M., Coyle, J. T., Hedreen, J. C. 1983. Topographic analysis of the innervation of the rat neocortex and hippocampus by the basal forebrain cholinergic system. J. Comp. Neurol. 217:103– 21
Mitchell, I. J., Clarke, C. E., Boyce, S., Robertson, R. G., Peggs, D., et al. 1989. Neural mechanisms underlying Parkinsonian symptoms based upon regional uptake of 2-deoxyglucose in monkeys exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine. Neuroscience 32: 213–26
Mocchetti, I. , Schwartz, J . P., Costa, E. 1985. Use of mRNA hybridization and radioimmunoassay to study mechanisms of. drug-induced accumulation of enkephalins in rat brain structures. Mot. Pharmacal. 28: 86–91
Monsma, F. J., McVittie, L. D., Gerfen, C.R., Mahan, L. C., Sibley, D. R. 1989. Alternative RNA splicing produces multiple D2 dopamine receptors. Nature 342: 926–29
Monsma, F. J., McVittie, L. D., Gerfen, C. R., Mahan, L. C., Sibley, D. R. 1990. Molecular cloning and expression of a D1 dopamine receptor linked to adenylyl cyclase activation. Proc. Natl. Acad. Sci. USA 87: 6723–27
Nakanishi, H., Kita, H., Kitai, S. T. 1988. An N-methyl-D-aspartate receptor mediated excitatory postsynaptic potential evoked in subthalamic neurons in an in vitro slice preparation in the rat. Neurosci. Lett. 95: 130–36
Nauta, W. J. H., Smith, G. P., Faull, R. L.M., Domesick, V. B. 1978. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience 3: 385–401
Normand, E., Popovich, T., Onteniente; B., Fellmann, D., Piatier-Tonneau, D., et al. 1988. Dopaminergic neurons of the substantia nigra modulate preproenkephalin gene expression in rat striatal neurons, Brain Res. 439: 39–46
Park, M. R., Lighthall, J. W., Kitai, S. T. 1980. Recurrent inhibition in the rat neostriatum. Brain Res. 194: 359–69
Penny, G. R., Afsharpour, S., Kitai, S. T. 1986. The glutamate decarboxylasw-, leucine enkephalin-, methionine enkephalinand substance -immunoreactive neurons in the neostriatum of the rat and cat: Evidence for partial population overlap. Neuroscience 17: 1011–45
Ragsdale, C. W. J., Graybiel, A. M. 1981. The fronto–striatal projection in the cat and monkey and its relationship to inhomogeneities established by acetylcholinesterase histochemistry. Brain Res. 208:259–66
Ragsdale, C. W. J., Graybiel, A. M. 1988. Fibers from the basolateral nucleus of the amygdala selectively innervate striosomes in the caudate nucleus of the cat. J. Comp. Neurol. 269: 506–22
Richfield, E. K., Penney, J. B., Young, A. B. 1989. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience 30: 767–77
Robertson, G. S., Vincent, S. R., Fibiger, H.C. 1990 Striatonigral projection neurons contain DI dopamine receptor-activated c-fos. Brain Res. 523: 288–90
Royce, G. J. 1982. Laminar origin of cortical neurons which project upon the caudate nucleus: A horseradish peroxidase investigation in the cat. J. Comp. Neurol. 205: 8–29
Royce, G. J., Bromley, S. 1984. Fluorescent double labeling studies of thalamostriatal and corticostriatal neurons. In The Basal Ganglia, ed. J. S, McKenzie, R. E. Kemm, L. N. Wilcock, pp. 131–46. New York: Plenum
Saper, C. B. 1984. Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus, J. Comp. Neurol. 222: 313–42
Sivam, S. P., Strunk, C., Smith, D. R., Hong, J.-S. 1986. Preproenkephalin-A gene regulation in the rat striatum: Influence of lithium and haloperidol. Mol. Pharmacol. 30: 186–191
Smith, Y., Bolam, J.P., Krosigk, M.V. 1990. Topographical and synaptic organization of the GABA-containing pallidosubthalamic projection in the rat. Eur. J. Neurosci 2: 500–11
Sokoloff, P., Giros, B., Martres, M.-P., Bothenet, M.-L., Schwarz, J.-C. 1990. Molecular cloning and characterization of a novel dopamice receptor 9D3) as a target fro neuroleptics. Nature 347: 146.
Somogyi, P., Bolam, J. P., Smith, A. D. 1981. Monosynaptic cortical input and local axon collaterals of identified striatonigral neurons. A light and electron mciroscopic study using the Goldi-peroxidase-degeneration procedure. J. Comp. Neurol 195: 567–84
Spencer, H. J. 1976. Antagonism of cortical excitation of striatal neurons by glutamic acid diethylester: Evidence for gluatmic acid as an excitatory transmitter in the rat striatum. Brain Res. 102: 91–101
Stoof, J. C., Kebabian, J. W. 1981. Opposing roles for the D-1 and D-2 dopamine receptors in efflux of cAMP from rat neostriatum. Nature 294: 366–68
Stoof, J. C., Kebabian, J. W., 1982. Independent in vitro regulation by the D2 dopamine receptor of dopamine-stimulated efflux of cyclic AMP and K+ -stimulated release of acetylcholine from rat neostriatum. Btrain Res. 250: 263–70
Sunahara, R. K. Nature 347: 80–83
Swanson, L. W. 1987. Limbic system. In Encyclopedia of Neuroscience, ed. G. Adelman, pp. 589–91. Boston: Birkhauser
Tang, F., Costa, E., Schwartz, J.P. 1983. Proc Natl. Acad. Sci. USA 80: 3841–44
Trugman, J. M., Wooten, G. F. 1987. J. Neurosci. 7: 2927–35
Undie, A. S., Friedman, E. 1990. j. Phamacol. Exp. Ther. 253: 987–92
Vincent, S. R., Hokfelt, T., Christensson, I., Terenius, L. 1982. Eur. J. Phamacol. 85: 251–52
Vincent, S. R. Johansson, O. 1983. J. Comp. Neurol. 217: 252–63
Voorn, P., Groenewegen, H., Gerfen, D. R. 1989. J. Comp. Neurol. 289: 189–201Walters, J.R., Bergstrom, D.. A., Carlson, J. H., Chase, T. N. Braun , A. R., 1987. D1 dopamice receptor activation required for postsynatpic expression of D2 agonist postsynaptic expression of the D2 agonist effects. Science 236: 719–22
Webster, K. E., 1961. Cortico-striate interrelations in the albino rat. J. Anat. 95: 532–44
Weiner, D. M., Levey, A. I., Brann, M. R. 1990. Proc. Natl. Acad. Sci. USA 87: 7050–54
Wilson, C. J. 1981. Brain Res. 220: 67–80
Wilson, C. J. 1987. J. Comp. Neurol. 263: 567–80
Wilson, C. J., Chang, H. T., Kitai, S. T. 1990. J. Neurosci 10: 508–19
Wilson, C. J., Groves, P. M. 1980. J. Comp. Neurol. 194: 599–615
Yokota, Y., Sasai, Y., Tanaka, K., Fujiwara, T., Tsuchida, K., et al., 1989. J. Biol. Chem. 264: 17649–52
Young, W. S. III, Bonner, T. I., Brann, M. R. 1986. Proc. Natl. Acad. Sci. USA 83: 9827–31
Zhou, Q. -Y., Grandy, D. K., Thambi, L., Kushner, J. A., Tol, H. H. M. V., et al. 1990. Clonging and expression of human and rat D1 dopamine receptors. nature 347: 76–80.
Since 16 May 2000
Put on the WWW by RW Williams and D. S. with permission from Chip Gerfen.